Cell Therapy Development: End-to-End solution
Standardize your process from Research and Development to Cell Manufacturing
CAR-T Therapy
In the past twenty years, T cell-based immunotherapies have greatly revolutionized the field of cancer treatment and enormous efforts have been devoted to developing new drugs as well as improving the efficiency of manufacturing processes. At AC, we provide researchers with technological solutions promising flexibility and scalability.
γδ T cells, the minor subsets of T cells hold promise for adoptive immunotherapy through their unique reactivity to bacteria, viruses, and tumours. However, because they constitute a small fraction (1–5%) of the peripheral T-cell pool, the isolation of γδ T cells requires a highly efficient, yet gentle isolation workflow.
Stem Cell Therapy
Multipotent haematopoietic stem cell (HSC) therapy is a popular technique with a range of promising health benefits. One of the recognized challenges is the ability to quickly obtain a sufficient number of desired cell types for transplantation, with high purity. We address it through an easy method to isolate CD34+ cells from fresh or mobilized blood without RBC lysis and density gradient centrifugation.
How we support you
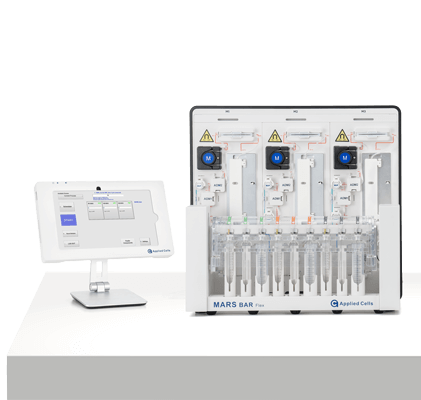
MARS® Platform In R&D
MARS® Bar Flex
RUO
Discovery
HIGH PURITY AND RECOVERY

FLEXIBILITY
variety of sample types
Custom programs

HIGH SPEED
MARS® Platform For Scale-Up
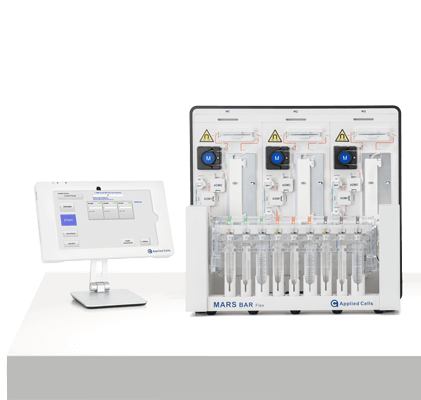
MARS® Bar Flex
RUO
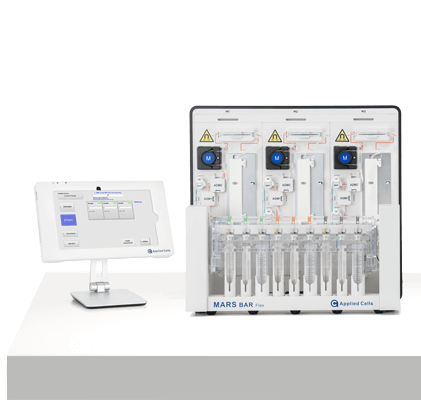
Discovery
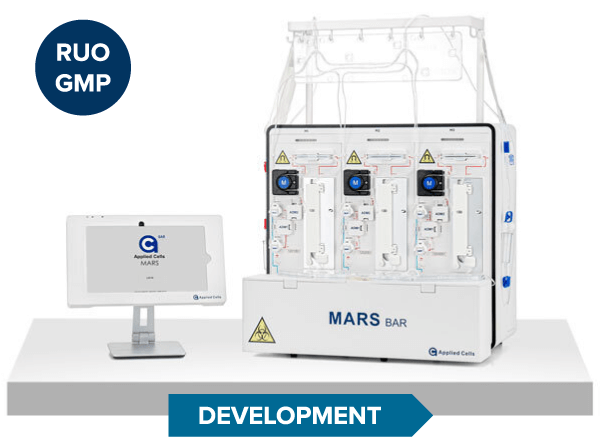
MARS® Bar
RUO
GMP

Development

EASY UPSCALING
Through consistent protocols across the platform

AUTOMATED
Reproducibility in batches

ECONOMICAL
MARS® Platform From Lab Bench to Clean Room
MARS® Bar Flex
RUO
Discovery
MARS® Bar
RUO
GMP

Development
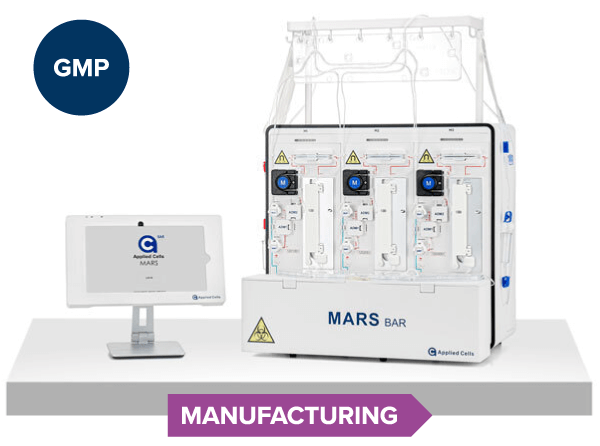
MARS® Bar
ISO13485
GMP

Manufacturing
MARS® Bar Flex

MARS® Bar
MARS® Bar
ISO13485
Our Team
Our main drive is to help researchers achieve their goals, and transfer knowledge and experience, opening doors to new solutions.
Whether we are with a small research lab or supporting a cell manufacturing company, we are always looking for a way to innovate, improve the process and identify new opportunities. Our focus has allowed us to work on fascinating projects in the USA, Europe and Asia, and has led us to establish this end-to-end solution.
Every day we challenge ourselves to push the limits of what’s possible in MARS® platform development. Each team member and business partner brings a unique blend of talent, passion and expertise to the table, and we all share the values of the research community.

Our technology
MARS® Bar is an in-flow immunomagnetic cell isolation platform. It doesn’t require magnetic columns and enables the sterile collection of both positive and negative fractions in separate bags.The system is manufactured and tested in an ISO13485 compliant facility, in compliance with CE (Conformité Européenne). The platform enables an easy and flexible transition from RUO process development to commercial manufacturing of GMP-compliant Advanced Therapy Medicinal Products, including cell and gene therapy.


