Cell Therapy Development Applications
Automated Cell Isolation for a variety of Cell Therapy types
TCR Cell Therapy
TCR Cell Therapy has gained popularity due to the range of possible treatments for cancer it could offer. γδ T-cells have been shown to constitute promising effector cell compartments for cancer immunotherapy, because of their ability to recognize and kill transformed cell without dependending on HLA-antigen presentation.
Although published protocols for the isolation of a large portion of TCR cells in vitro exist, the purity of the final preparation between different donors is often heterogeneous. One solution is the use of column-free, immunomagnetic TCR α/β+ depletion, as demonstrated using MARS Bar Platform.
TCR therapy is still a relatively new, experimental treatment. We are supporting Cell Therapy development to help realize the full potential of TCR therapy and revolutionize cancer treatment.
Together with STEMCELL Technologies, we have developed a MARS MAG Premium Line of reagents, offering kits for single-step TCR α/β+depletion.
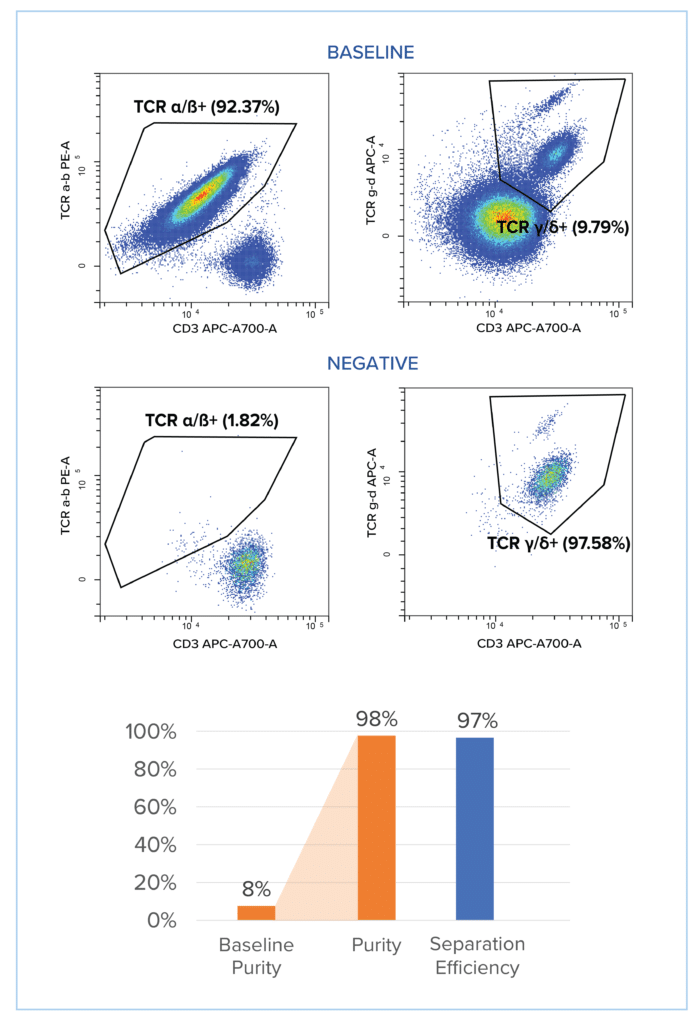
Figure on the right: TCR Cell Therapy development: TCR γ/δ+isolation through TCR α/β+depletion >99.8% with separation efficiency exceeding 97% from leukapheresis product.

TCR γ/δ+ separation through TCR α/ß+ depletion from PBMC with a column-free immuno-magnetic workflow
NK Cell Therapy
NK Cell Therapy utilizes engineered Natural Killer cells to target tumor cells and other diseases, and it is one of the most dynamically developing therapies. It involves isolation of NK cells from patient or other donot, NK cell modification and infusing it into patient’s blood stream for treatment. NK Cell Therapy has shown promising results in treating certain types of cancer, for example Leukemia, Lymphoma, or Multiple Myeloma.
Some of the key advantages of the NK Cell Therapy are increased therapy safety, multiple cytotoxicity mechanisms, and potential of the engineered NK cells to infiltrate into solid tumors. The success of NK Cell Therapy depends on the number and quality of NK cells used. MARS Bar platform enables the isolation of the NK cells from starting samples including Apheresis products and Whole Blood without the need of PBMC preparation.
Our reagent MARS MAG Line and MARS MAG Premium Lines of reagents offer flexible beads and cocktails for positive or negative NK cell isolation for NK cell therapy development.
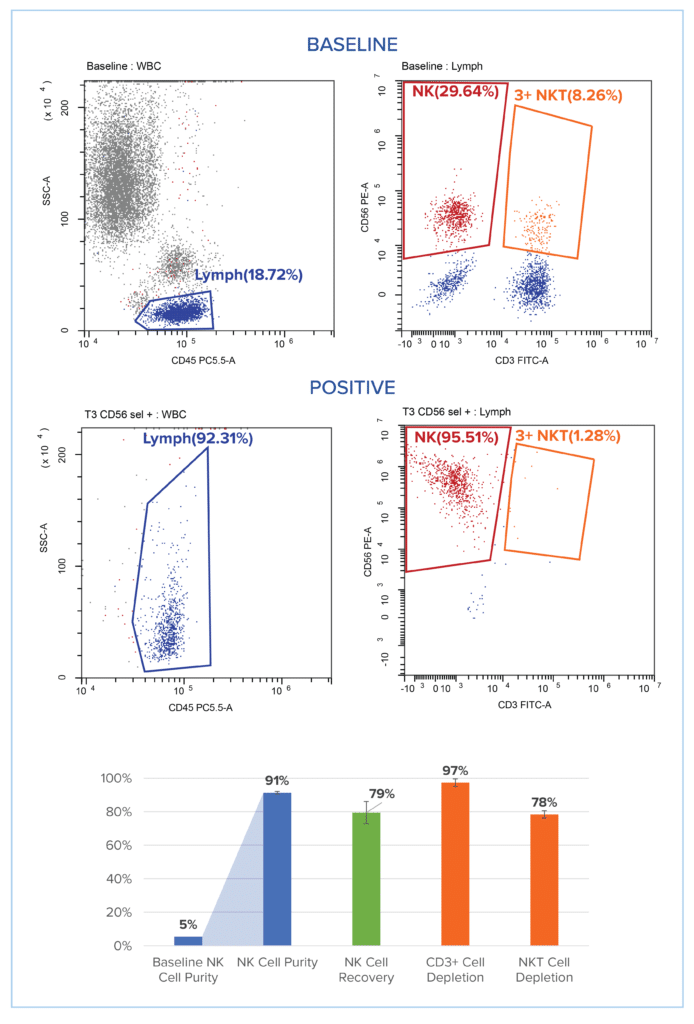
Figure on the right: CD56+/CD3 – Cell Enrichment straight from Peripheral Blood Samples with >90% purity and high recovery

Stem Cell Therapy
Stem Cell Therapy involves the use of CD34+ cells with the potential to develop into different types of cells in the body. Examples of different types of stel cells include embryonic stem cells, induced pluripotent stem cells, or adult stem cells, all of which have unique potential for their use in therapy.
Intensive stem cell therapy development promises new cutting-edge treatments against diseases including some types of cancer, neurological disorders, cardiovascular diseases, wounds, and others.
The use of Stem cells in therapy is still a relatively new field, and intensive research is being conducted to fully understand its potential benefits and risks. MARS® Platform allows isolation of CD34+cells with high recovery, allowing for an efficient cell therapy development with a smaller input sample volume.
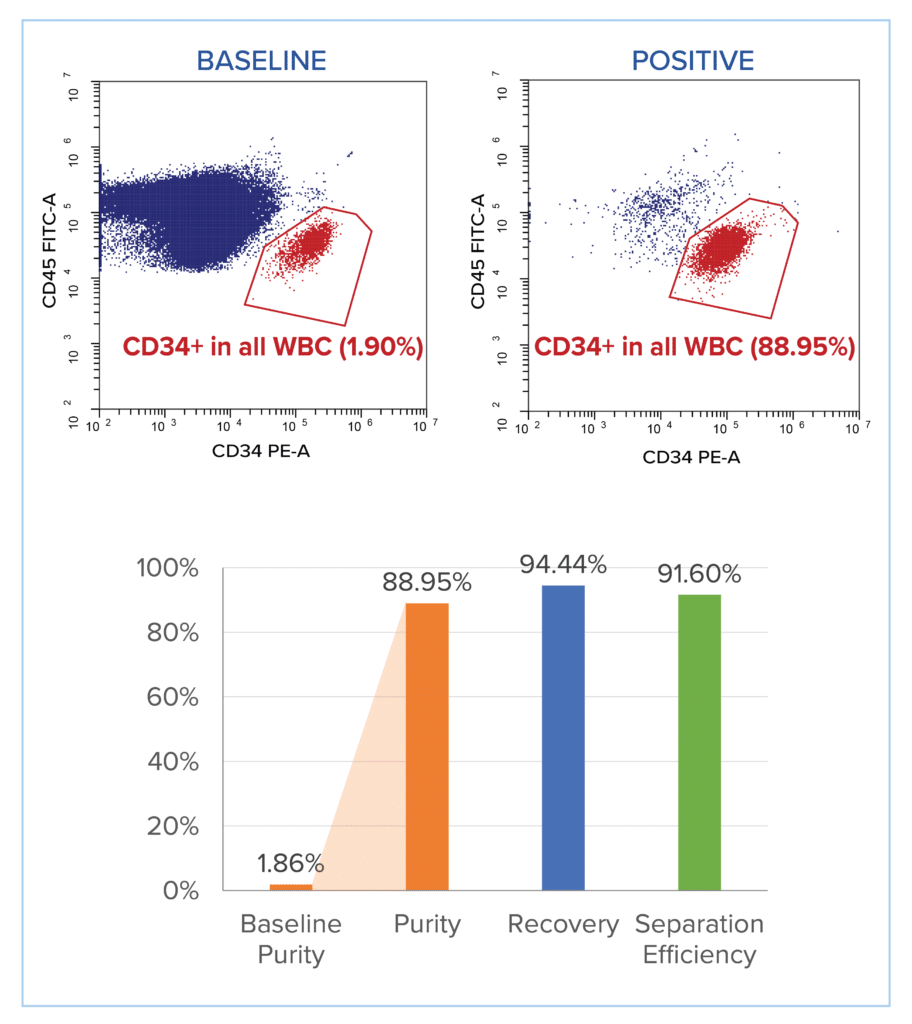
Figure on the right: Hematopoietic Stem cell isolation optimization with an easy and cost-effective protocol: single-pass CD34+ HSC enrichment from baseline 1.9% purity to 88.95% purity and recovery >94.4%

About MARS® Bar Platform
MARS® Bar platform supports your cell development process from the lab bench to the clean room:
- from: small-scale assay optimization processing 3 samples in parallel
- through: large-scale RUO validation
- to: sterile cell manufacturing with MARS® Bar system manufactured and tested in an ISO13485-compliant facility.
With the propriety in-flow immunomagnetic cell isolation technology, MARS® users are not limited by column capacity. Additionally, the MARS® Bar system allows for the collection of both positive and negative fractions in separate tubes or bags, providing the users with the option to fully utilize all the collected fractions.
Explore other MARS® Platform Applications
Target Cell Isolation
Choose one of MARS gentle, centrifuge-free and Ficoll-free workflows for immunoselection of TIL, T Cells, NK cells Neutrophils or Stem cells.
Cell and Nuclei Enrichment
Easy and fast debris removal to feed genomic platforms with enriched cells from samples including Whole Blood, tumor, PMBC, solid tissue and many others.