Unlocking the potential of CAR T cell therapy with
MARS AtlasTM
Revolutionizing Therapy Development
Autologous cell therapy using genetically modified T cells expressing a chimeric antigen receptor (CAR) shows promising results in treating cancers. However, accessibility remains limited due to high costs and lengthy manufacturing processes. Conventional CAR T cell manufacturing involves large cell numbers, numerous steps, and long process time, leading to high costs and limited manufacturing capacity. In contrast, the MARS AtlasTM platform streamlines the process, offering rapid and automated manufacturing for high potency CAR-T cells.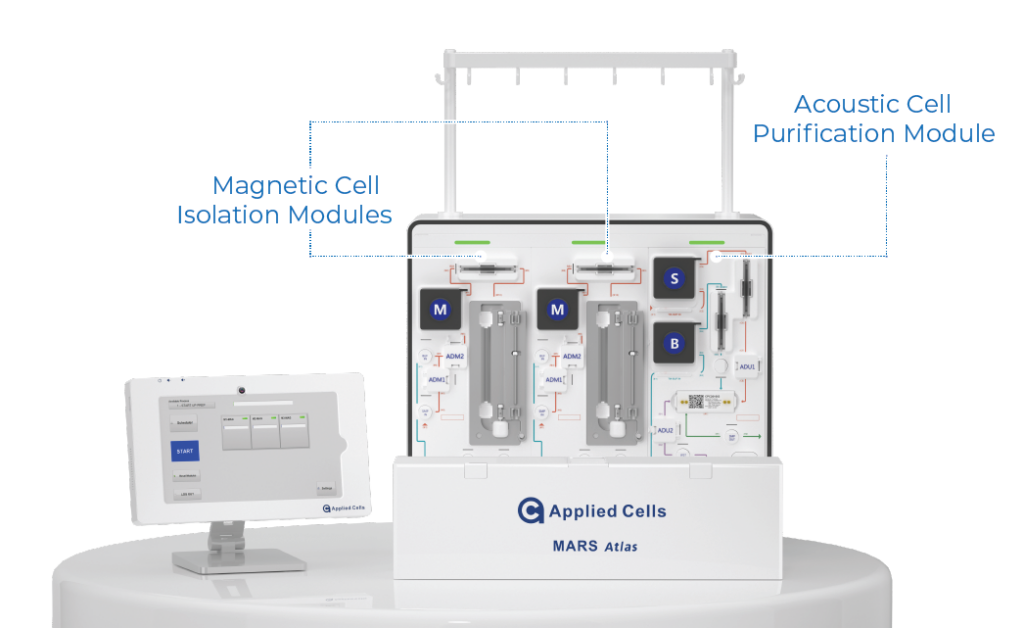
Rapid CAR-T Manufacturing
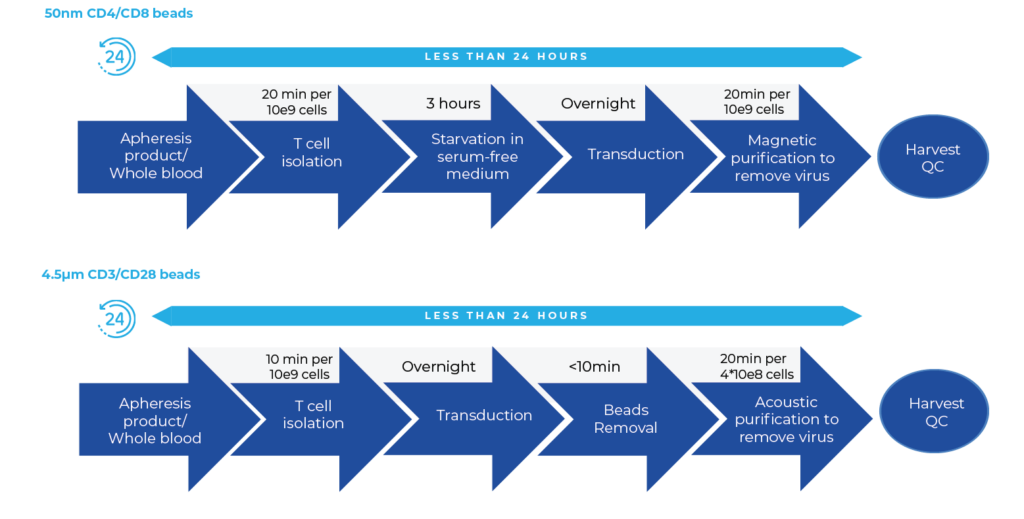
Positive Selection MAG → CM → MAG
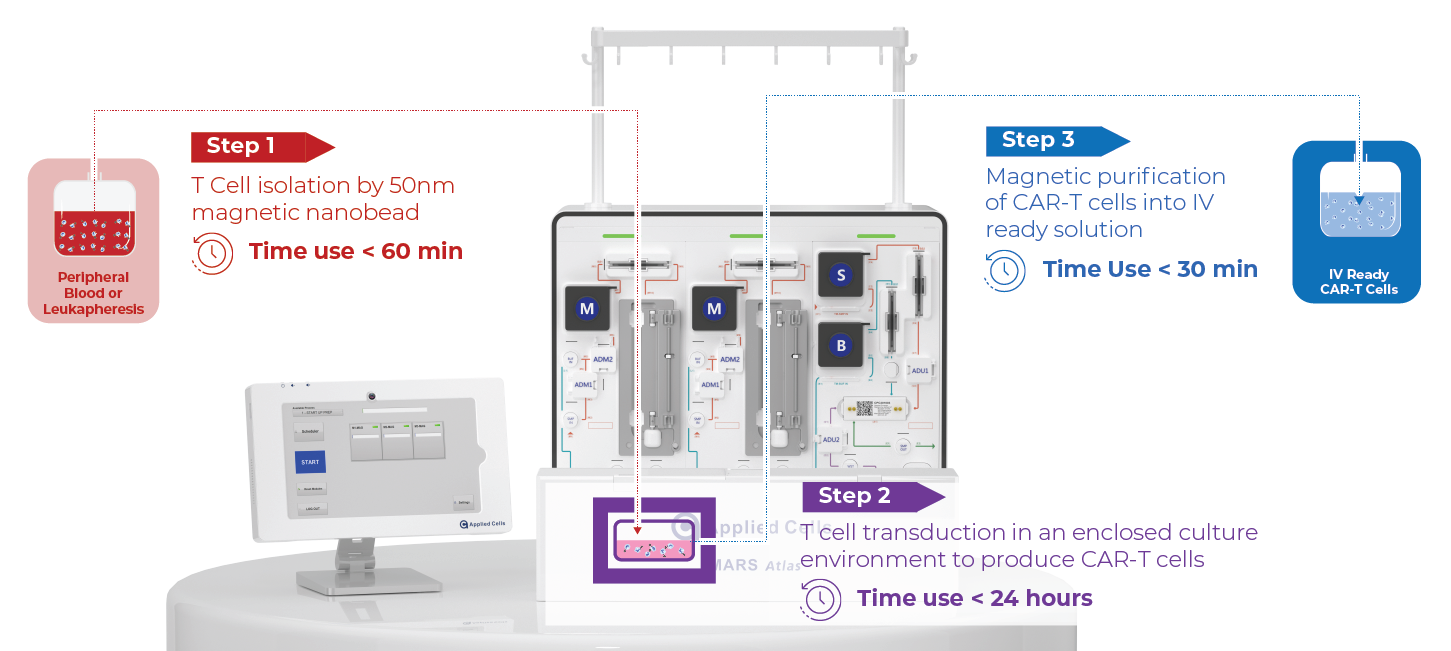
T Cell Isolation
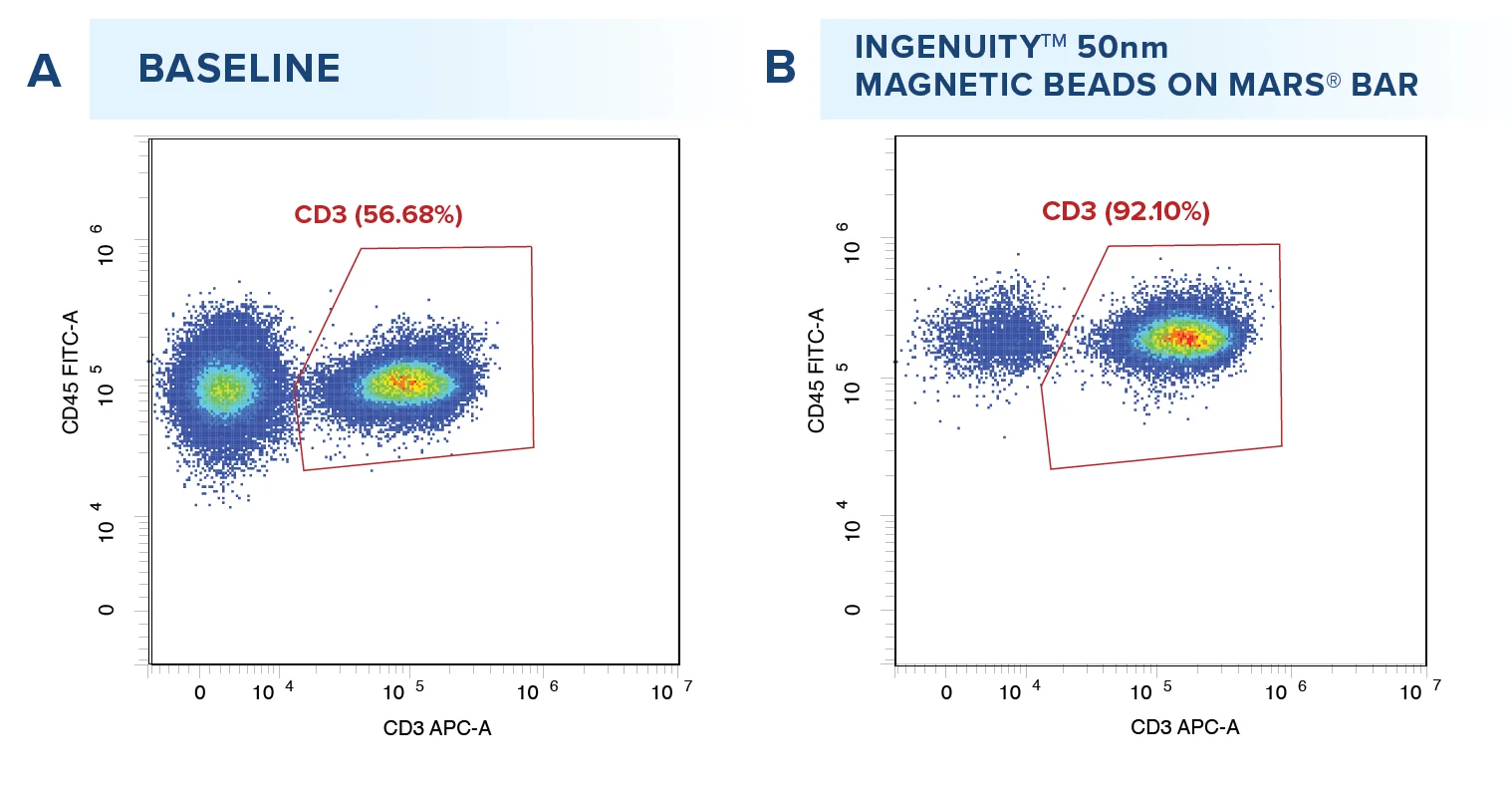
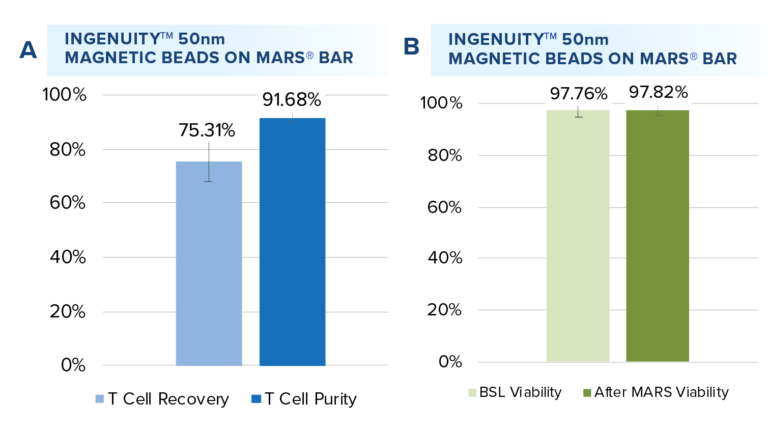
Starting from an initial average T cell purity of 49.92%, T cell positive selection was performed with the IngenuityTM 50nm CD4 and CD8 beads, resulting in an average recovery rate of 75.31% T cells with a mean purity of 91.68% (A, n= 12 samples). Viability assessment before and after positive selection (B) revealed no significant impact (P<0.5) on cell viability, ensuring the integrity of T cells across the isolation process.
Cell Purification
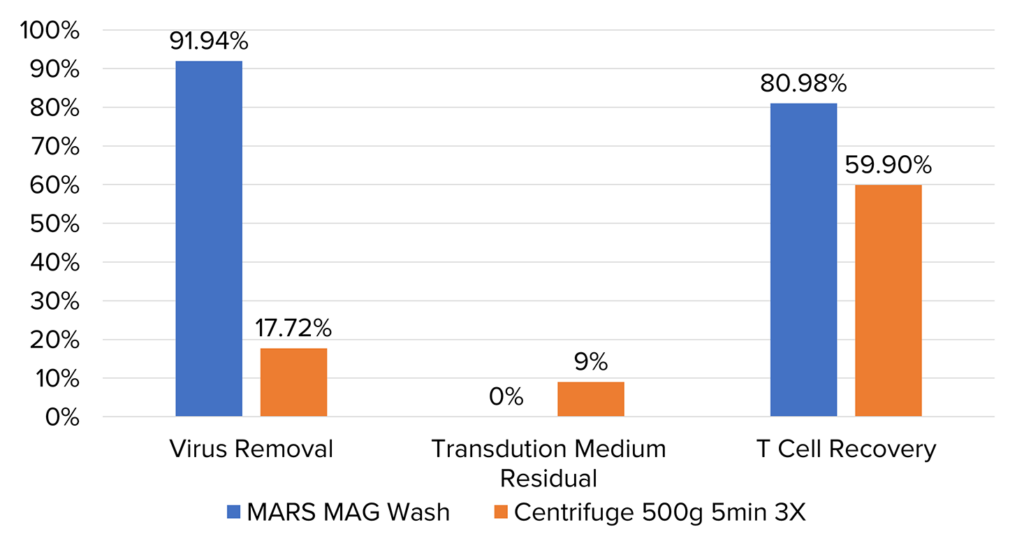
Comparing MARS® Mag wash to centrifugation after transduction: conducted 24 hours post-transduction initiation, we evaluated the effectiveness of both methods in removing free virus and polybrene. MARS® Mag wash was performed once, while centrifugation was carried out three times at 500g for 5 minutes each. Evaluation was conducted using flow cytometry to measure virus removal and T cell recovery, and residual transduction medium was quantified using a fluorescent plate reader. Results indicated that MARS® Mag wash achieved a 91.94% virus removal rate and an 80.98% T cell recovery rate, with no residual medium remaining.
Viral Transduction and Expression
Positive Selection MAG → CM → MAG → CPC
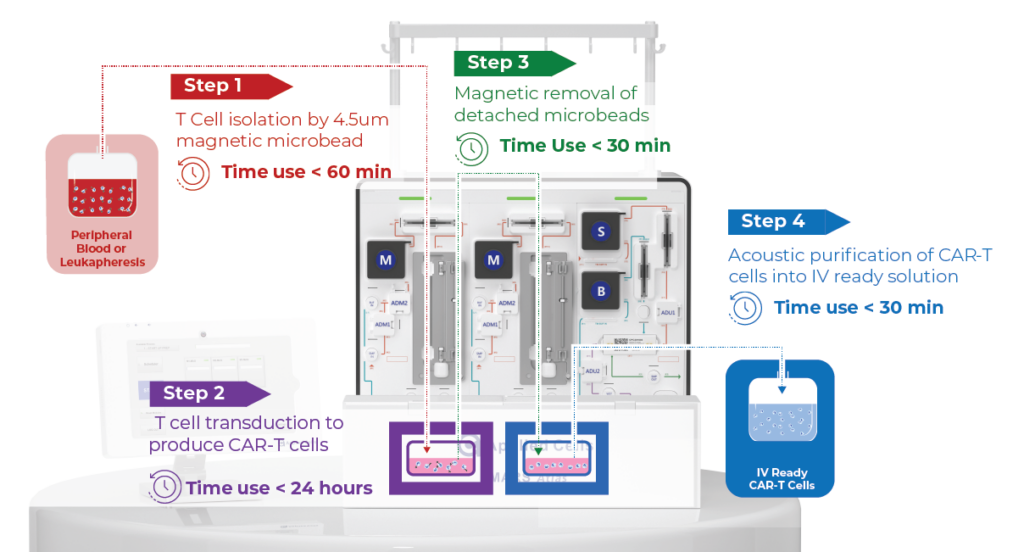
T Cell Isolation
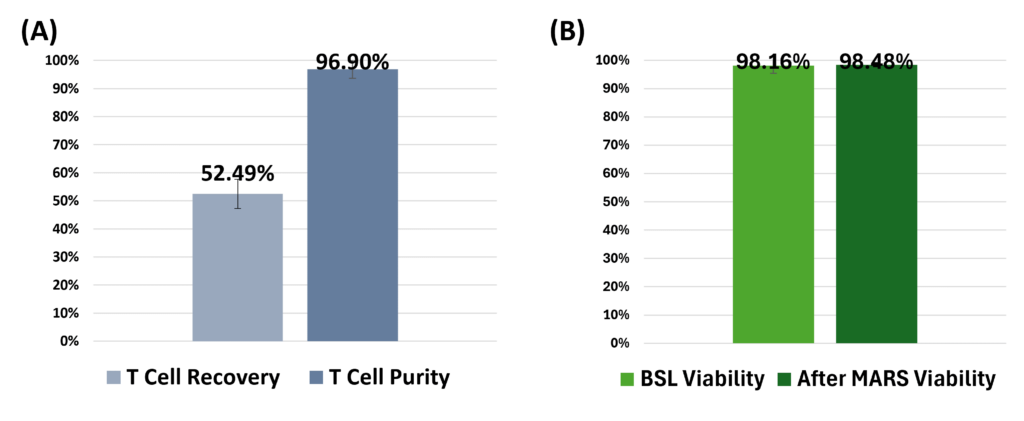

Efficient Isolation of T Cells from Whole Blood Using the MARS AtlasTM MAG module, with Preserved Viability: (A) T cell positive selection utilizing IngenuityTM CD3/CD28 microbeads at a 1:1 bead to T cell ratio resulted in an average recovery of 52.49% T cells with an average purity of 96.90%, from an initial average T cell purity of 25.10%. Data collected from n=3 healthy donor samples. (B) Viability of isolated T cells remained unaffected before and after positive selection with IngenuityTM CD3/CD28 microbeads (P<0.5). (C) Whole blood sample prior to separation, and (D) Enriched T cells following positive selection with IngenuityTM CD3/CD28 microbeads are depicted, highlighting the efficacy of the MARS AtlasTM platform in maintaining high purity during T cell isolation while preserving cell viability.
Viral Transduction
Cell Purification
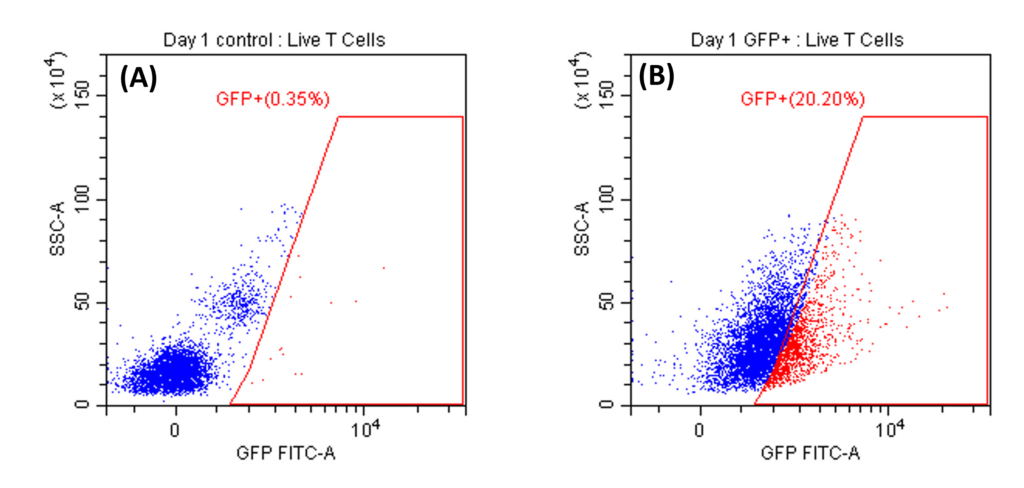
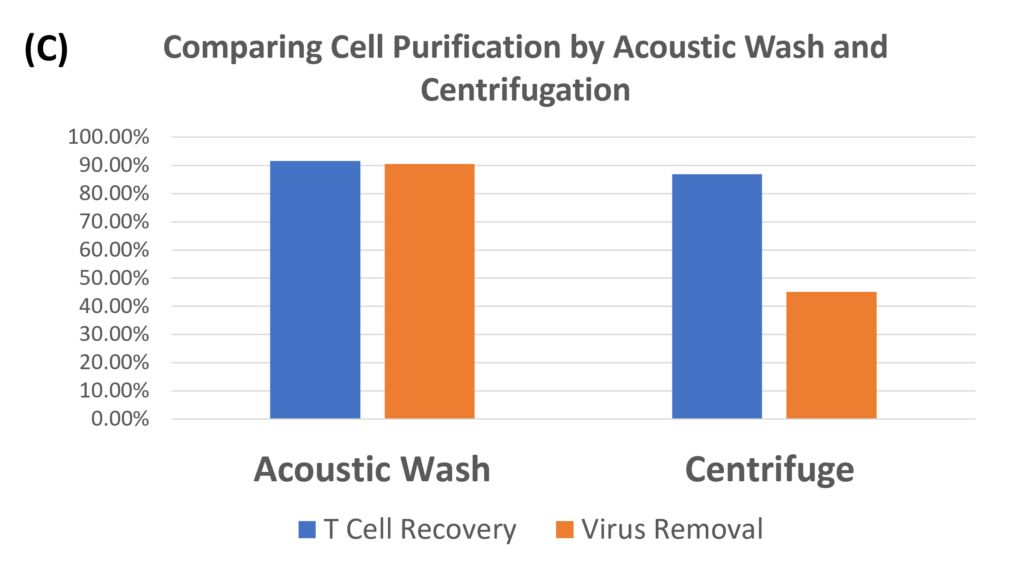
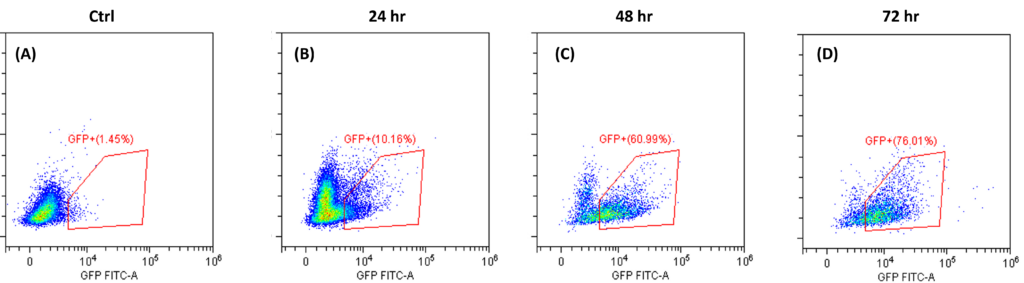
Transduction Results and Efficient Removal of Free Virus; (A) and (B) illustrate the 24-hour transduction outcomes using the identical protocol detailed in Figure 4. Following a 24-hour incubation period, 20% of T cells became GFP positive. (C) Efficient removal of free virus is demonstrated. Employing the acoustic module to purify T cells post-de-beading yields a 90% T cell recovery alongside 90% virus removal with a single pass through the acoustic chip. In comparison, a centrifuge wash at 500g for three cycles results in 80% cell recovery and 50% virus removal. Free beads were completely removed through the bead removal process; data not displayed. This highlights the superior efficacy of the acoustic module in achieving both high cell recovery rates and substantial virus removal, crucial for optimizing experimental outcomes.
Explore other MARS® Platform Applications
Target Cell Isolation
Choose one of MARS gentle, centrifuge-free and Ficoll-free workflows for immunoselection of TIL, T Cells, NK cells Neutrophils or Stem cells.
Explore other MARS® Platform Applications
Cell and Nuclei Enrichment
Easy and fast debris removal to feed genomic platforms with enriched cells from samples including Whole Blood, tumor, PMBC, solid tissue and many others.