CD138 Plasma Cell Enrichment for FISH*
*For Research Use Only - not for use in therapeutic or diagnostic procedures
Enhancing diagnostic precision in multiple myeloma
New solutions that improve the diagnostic precision of multiple myeloma are key for advancing disease research, improving patient diagnostics, treatment and care. We offer streamlined workflow for isolating CD138+ plasma cells for multiple myeloma (MM) diagnosis and cytogenetic analysis. Using MARS MAG Premium line CD138 Positive Selection Kit and the MARS BAR Flex platform, we achieved high recovery rates, purity, and a higher detection rate of abnormal signals compared to conventional methods.
Selection of CD138+ cells is quick, robust and requires minimal hands-on time using MARS Bar Flex.
CD138+ cells separation from peripheral whole blood
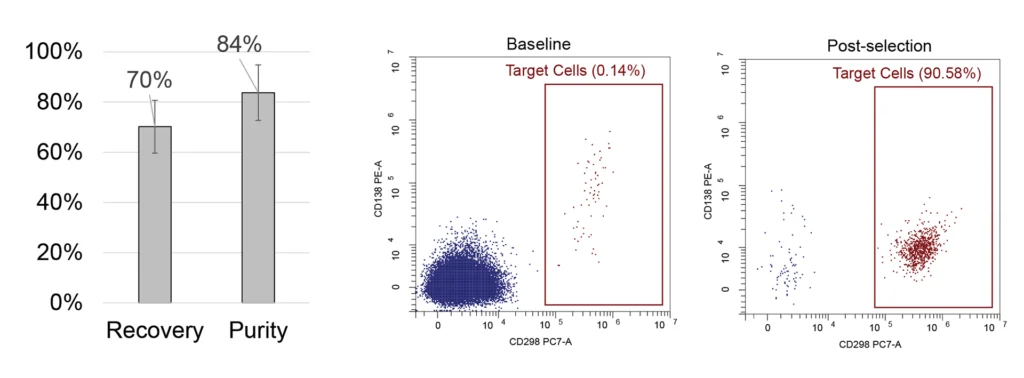
Whole blood samples were spiked in with CD138+ cells from MM1.S cell line to mimic MRD, PBMC were enriched with Ficoll and processed with MARS platform. We achieved an average purity of 84% of target cells with an 11% standard deviation, starting from the initial 0.1-0.5% in the input samples, and the recovery rate of 70% with an 11% standard deviation. Study included 10 samples from 6 donors.
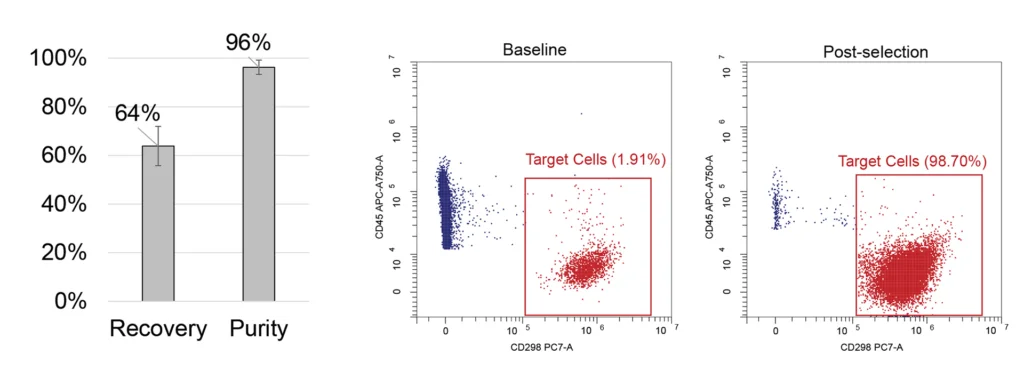
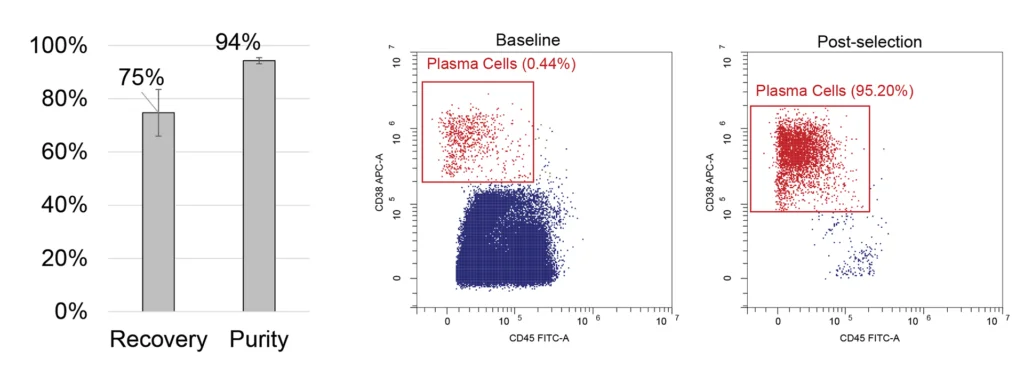
Selection of CD138+ cells from bone marrow
Upper Panel: PBMC samples were spiked in with MM1.S cell line cells to mimic bone marrow and processed with MARS BAR Flex to isolate CD138+ cells. We achieved an average recovery rate of 64% with an 8% standard deviation and an increase in the purity of target cells to 96% from the initial 1%; n=6 samples from 2 donors.
Lower panel: CD138+ plasma cells were selected from healthy whole bone marrow. Across 4 samples from a single healthy donor, we achieved an average recovery rate of 75% with a 9% standard deviation and target cell purity increase reaching 94% from an initial 0.44% (1% standard deviation).
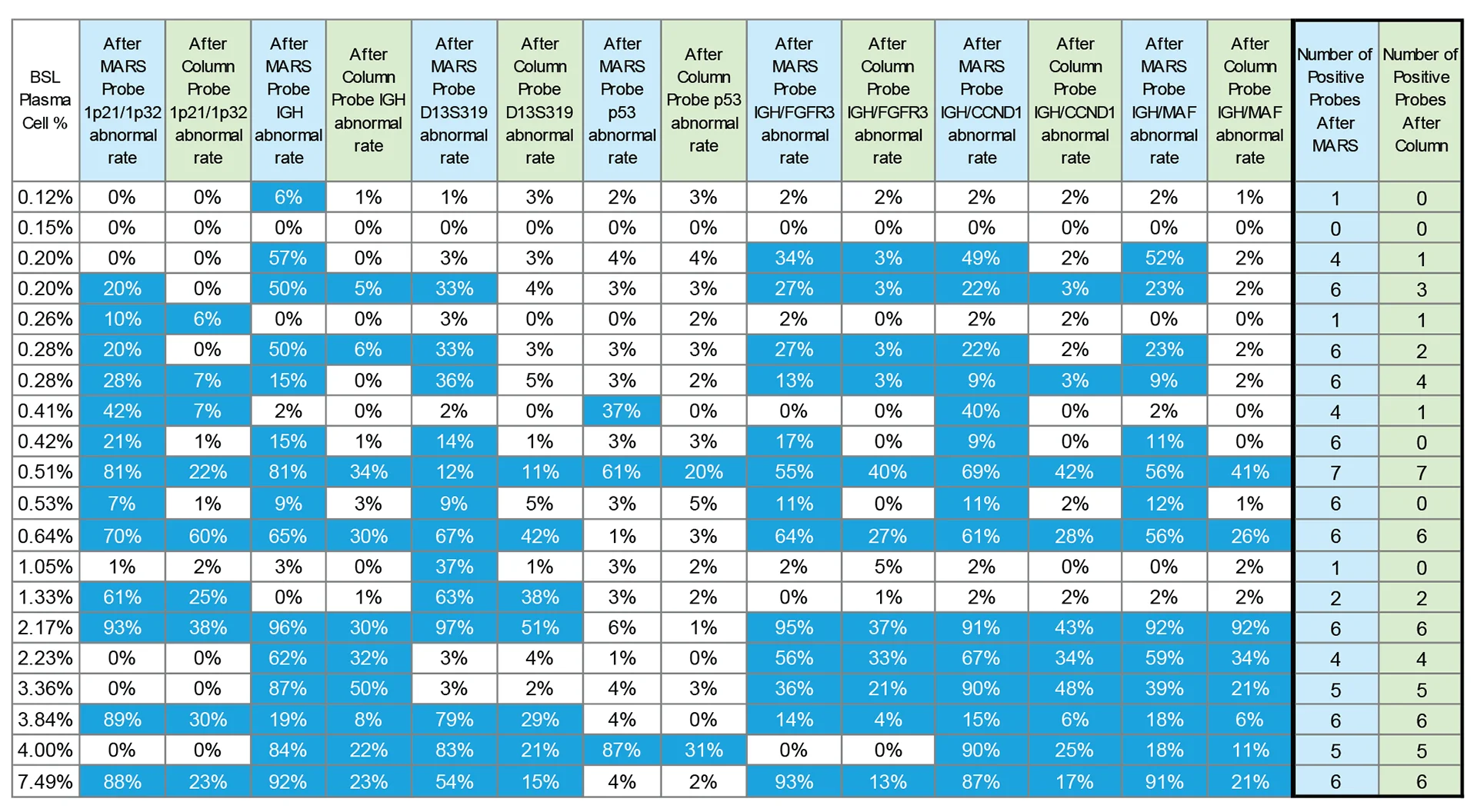
MARS improves the abnormal signal detection using FISH in multiple myeloma patients
Twenty bone marrow samples from multiple myeloma patients with varying plasma cell frequencies were enriched using either the MARS Bar platform or a column-based magnetic enrichment method*. Plasma cells were enriched directly from bone marrow without Ficoll preparation. Next, we used fluorescence in situ hybridization (FISH) to detect abnormal signals from different probes: 1p21/1p32 (positive threshold 5%), IGH (positive threshold 5%), D13S319 (positive threshold 8%), p53 (positive threshold 8%), IGH/FGFR3 (positive threshold 3%), IGH/CCND1 (positive threshold 3%), and IGH/MAF (positive threshold 3%).
The table above summarizes the percentages of abnormal cells detected by each method, and table cells meeting or exceeding the respective threshold highlighted in blue and marked as positive.
The data demonstrates, that the percentage of cytogenetically abnormal cells detected by FISH was consistently higher post MARS enrichment in contrast to the column-based method. In nine cases, MARS detected more positive probes than the column-based method.
*The counts of positive probes for both methods are summarized in the final two columns (highlighted in blue and green). Note that for patients with baseline plasma cell percentages exceeding 1.1%, the positive probe counts were identical for both MARS and the current method, however, among patients with less than 1.1% baseline plasma cells, only 4 showed identical positive probe counts between methods.
MARS® Platform: Pioneering the Future of Plasma Cell Enrichment
The MARS® platform emerges as a new solution to these challenges. Its unique in-flow technology, offers unparalleled flexibility in optimizing workflows while also ensuring unmatched purity and recovery rates.
What sets the MARS platform apart is its capability to directly isolate target cells — even those present in small starting percentages — from fresh samples, either peripheral blood or bone marrow. With its in-flow immuno-magnetic separation technology, the platform can well isolate CD138+ plasma cells straight from untreated bone marrow samples.
The MARS® platform is all about simplicity. Following the straightforward labeling protocol, the sample undergoes automated, seamless serial MARS® Immunomagnetic separation process. For the highest efficiency, the option to re-process samples through the magnetic channel to improve purity while maintaining recovery remains a significant advantage.
The Complex Landscape of Multiple Myeloma and the Crucial Role of FISH
Multiple myeloma, a complex and often aggressive neoplasm of plasma cells, stands as a profound challenge in the world of medicine. New data brings to light a concerning surge in global diagnoses and this trend highlights the pressing need for advanced diagnostic tools that can quickly identify the disease, thereby offering patients better prognostic outcomes. Important to this diagnostic evolution is the Fluorescence In Situ Hybridization, commonly known as FISH.
Renowned for its high sensitivity, FISH can identify even the subtlest of genetic irregularities, which highlights its crucial role in hematology and oncology. This technique has ushered in a transformative era in how medical practitioners interpret genetic variations within cells. Furthermore, its specificity ensures that patients receive not only a precise diagnosis but the one that’s well tailored, giving them a promising insight into their health status.
Yet, for all its advancements, the FISH technique can also be challenging. Multiple myeloma is unique among hematologic malignancies, characterized by a considerable portion of minimally proliferating malignant plasma cells. The scarcity of these cells in bone marrow aspirate further complicates cytogenetic analyses. One of the most significant hurdles is ensuring the high-quality enrichment of CD138 plasma cells, a cornerstone upon which the effectiveness and dependability of FISH rely. This underlines an urgent need for methods and technologies that can consistently deliver high-quality samples suitable for analysis.
Download White Paper