Making CAR-T more accessible: rethinking manufacturing economics
CAR-T cell therapy continues to demonstrate remarkable clinical potential, offering new options for patients with otherwise limited treatment pathways. Initially developed for hematologic malignancies such as diffuse large B-cell lymphoma and acute lymphoblastic leukemia, CAR-T approaches are now being developed for a broader range of solid tumors and autoimmune diseases, including lupus, myasthenia gravis, and multiple sclerosis.
The promise and the challenge
Yet, while these outcomes have advanced considerably, the economics of manufacturing remain a central consideration in determining how broadly these therapies can be delivered worldwide. Achieving equitable access will depend not only on scientific progress but also on how efficiently and sustainably CAR-T therapies can be produced and distributed.
The pricing landscape today
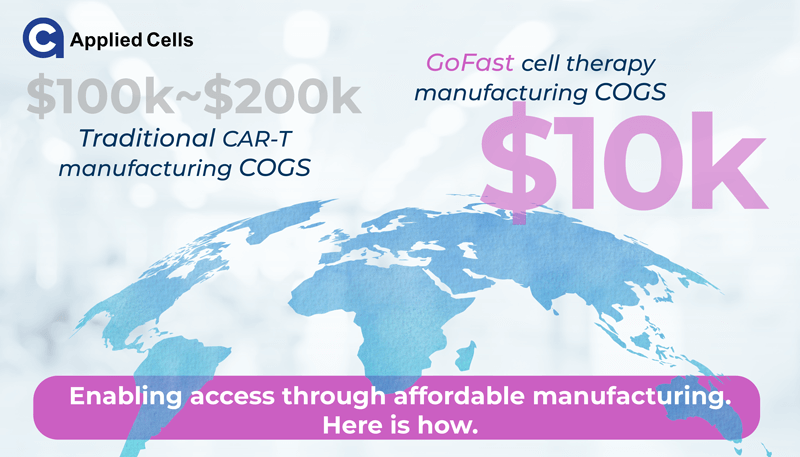
In high-income markets, commercial CAR-T therapies are typically priced between US $373,000 and $475,000 per treatment1. By comparison, in China, prices often range from US $150,000 – $450,000, while in India, reported treatment packages are between US $50,000 – $85,0002.
From a manufacturing perspective, the autologous CAR-T cost of goods sold (COGS) has been estimated at around US $95,000 per dose3. Even before factoring in logistics, facility overhead, and clinical margin, this baseline cost highlights the economic challenges of scaling such personalized therapies.
Why cost shapes access?
To understand affordability in a global context, health-economics analyses could compare therapy cost to national GDP per capita4:
- United States ≈ US $85k
- China ≈ US $13k
- India ≈ US $2,6k
A single CAR-T treatment priced at US $400 K represents roughly 167 × India’s GDP per capita, 30 × China’s, and 4.7 × the U.S. benchmark. At these levels, sustained reimbursement or self-pay adoption remains difficult.
If manufacturing costs drop below US $10 K per sample, overall pricing could begin to approach levels viewed as more compatible with national reimbursement frameworks, in line with earlier cost-effectiveness thresholds of roughly 1–3x GDP per capita per QALY.5.
How manufacturing cost influences therapy price?
Manufacturing represents one of the largest variables in final therapy cost. A 2023 analysis estimated that for Ciltacabtagene Autoleucel, non-acquisition costs averaged US $160,933, roughly 26 % of total patient cost6. When COGS exceeds US $95 K, downstream expenses can compound quickly. Efforts to make CAR-T therapies more widely accessible therefore often focus on innovations that can gradually reduce production time, material use, and labor intensity, without compromising quality or regulatory compliance.Pathways toward greater affordability
Global discussions around manufacturing reform commonly highlight several converging strategies:
- Automation and closed systems, with the goal to reduce manual labor and contamination risk.
- Standardized processes, helping ensure consistent quality and simplified validation.
- Regional manufacturing models, designed to shorten logistics chains and reduce cryopreservation or shipping requirements.
Collectively, these approaches aim to build a foundation for cost-efficient, scalable, and reproducible production, essential characteristics for global access.
Applied Cells’ approach to scalable manufacturing
Applied Cell’s MARS platforms are being developed with these same goals in mind: to help streamline workflows and enable higher throughput within compact infrastructures.
Toward lower COGS through high-throughput scalability
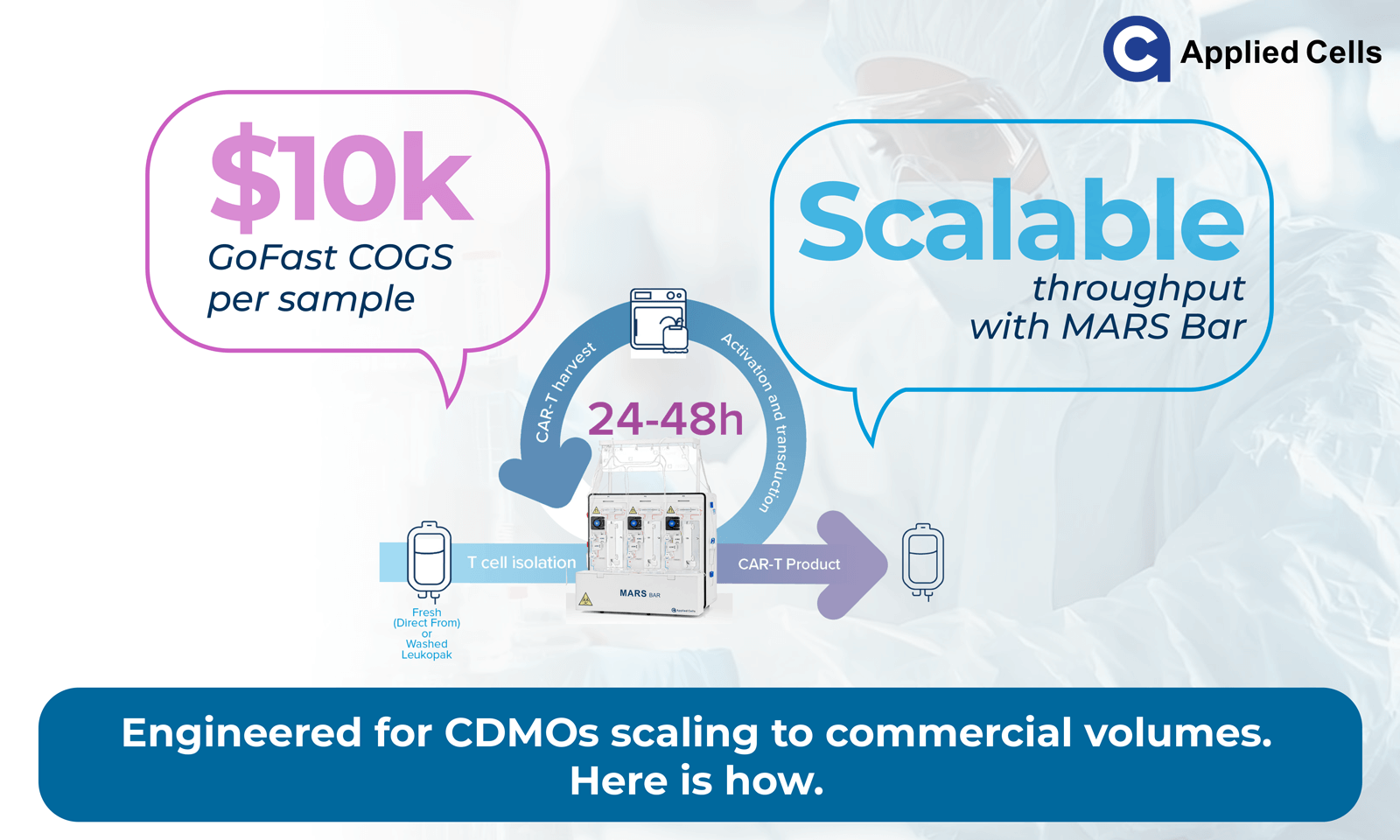
The MARS® Bar platform integrated with the GoFast™ workflow is designed to support COGS below US $10,000 per patient sample, representing a substantial step toward affordability. Being an innovative closed platform, MARS Bar offers the most cost-effective consumables and reagents. By completing production within approximately 72 hours, the process reduces media use, labor cost, and operator time compared with conventional multi-week expansions.
Intended outcome: a lower cost per sample that may translate into improved access and sustainability.Compact infrastructure and flexible throughput
A laboratory area of around 10 m², equipped with one MARS Bar and a CO₂ incubator, can process up to five samples per day, equating to an estimated 1,200 – 1,400 patient samples annually. A dual-unit setup can further increase operational continuity and space efficiency.
Such compact configurations could support regional manufacturing hubs, enabling multiple hospitals to share standardized capacity without major facility expansion.
Intended outcome: higher throughput within a smaller footprint, contributing to more predictable production economics.Operational simplicity and reproducibility
The GoFast™ process follows a three-step sequence:
• T-cell selection
• CAR transduction and incubation
• Harvest
With built-in automation, this design aims to minimize manual handling, reduce batch variability, and simplify validation steps, factors that may lead to lower operational overhead and faster turnaround times across decentralized sites.
Evolving examples in emerging markets
Local manufacturing initiatives are beginning to reshape CAR-T economics. In India, where GDP per capita is about US $2,400, locally produced CAR-T therapies have been reported at US $30 K – 50 K per dose7. In China, commercial therapies remain near RMB 1 million (~US $154 K) 8. Adoption of automated, high-throughput systems such as MARS Bar may offer a pathway to further reduce COGS below US $10 K per sample, supporting the long-term sustainability of regional production networks.
Why cost reduction aligns with access?
Lower manufacturing cost is more than an operational target, it is closely linked to health-equity outcomes. As production costs decline, therapies may become viable within national reimbursement frameworks, allowing treatment to reach broader patient populations.
Benefits of improved scenarios include:
- Expanded public coverage in middle-income economies.
- Decentralized manufacturing closer to treatment centers.
- Reinvestment capacity, freeing resources for ongoing R&D.
- Improved health equity, supported by more predictable and sustainable cost models.
Conclusion
CAR-T therapy continues to transform the treatment landscape for cancer and autoimmune disease alike. However, affordability remains one of the key determinants of how widely these benefits can be realized.
- Through advances in automation, standardization, and high-throughput scalability, new manufacturing platform MARS is helping redefine what cost-effective CAR-T production could look like.
- Applied Cells’ MARS® Bar and GoFast™ workflow illustrate a new approach, aiming to reduce COGS below US $10 K per sample and enable compact, scalable manufacturing footprints that support regional accessibility.
- As the field continues to evolve, such innovations may help bridge the gap between therapeutic promise and global access—bringing the benefits of CAR-T therapy closer to more patients, everywhere.
Connect with us to explore how GoFast™ can transform your cost model and operational efficiency.
References
- https://www.accc-cancer.org/docs/projects/car-t-cell-therapies/the-economics-of-car-t-cell-therapy.pdf?sfvrsn=e674d5f8_2
- Medical Tourism Co. (2025). CAR T-Cell Therapy for Cancer in India: Cost, Hospital & Approved Oncology Program
- https://www.sartorius.com/en/applications/cell-and-gene-therapy/cell-therapy/cell-therapy-guide/advanced-therapy-perspectives/what-will-it-take-to-realize-the-promise-and-potential-of-immune-cell-therapies-blog?
- https://data.worldbank.org
- Marseille, E., Larson, B., Kazi, D. S., Kahn, J. G., & Rosen, S. (2015). Thresholds for the cost–effectiveness of interventions: alternative approaches. Bulletin of the World Health Organization, 93(2), 118–124.
- Jagannath, Sundar, et al. “Component Costs of CAR-T Therapy in Addition to Treatment Acquisition Costs in Patients with Multiple Myeloma.” *Oncol Ther* 11, no. 2 (2023): 263–75.
- KPMG. (2025). The USD 30k CAR-T therapy: a future within reach?
Retrieved from https://assets.kpmg.com/content/dam/kpmgsites/ch/pdf/car-t-therapy.pdf.coredownload.inline.pdf - BioSpace. (2025). China Approves First Commercial CAR-T Cell Therapy. Retrieved from https://www.biospace.com/china-approves-first-commercial-car-t-cell-therapy
Disclaimer:
This article is provided for informational and educational purposes only. It is intended to share general insights into global trends in cell therapy development, affordability, and manufacturing innovation. Nothing contained herein should be interpreted as medical advice, clinical guidance, investment recommendation, or regulatory direction.
All figures, ratios, and cost estimates — including GDP per capita comparisons, affordability metrics, and cost-of-goods (COGS) thresholds — are approximate and intended for illustrative discussion only. These values may be based on publicly available economic data, rounded calculations, or generalized industry benchmarks, and should not be interpreted as definitive economic analyses or pricing commitments.
Product specifications, performance data, and cost targets related to the MARS® Bar and GoFast™ platforms reflect internal evaluations, prototype configurations, or vendor-reported design goals. Actual results may vary depending on implementation, scale, and local conditions. Applied Cells makes no representation or warranty, express or implied, regarding the completeness, accuracy, or currency of such information.
References to external studies or clinical outcomes (including CAR-T therapies and disease indications) are drawn from independent peer-reviewed publications and are not generated using Applied Cells products unless explicitly stated. MARS® Bar and GoFast™ platforms are for research use only and are not cleared, approved, or authorized by any regulatory agency for clinical use.
Applied Cells, Inc. reserves the right to modify product specifications, performance claims, or documentation at any time without notice. The content is provided “as is,” without warranties of any kind, and Applied Cells assumes no liability for the use, interpretation, or reliance upon the information presented.

