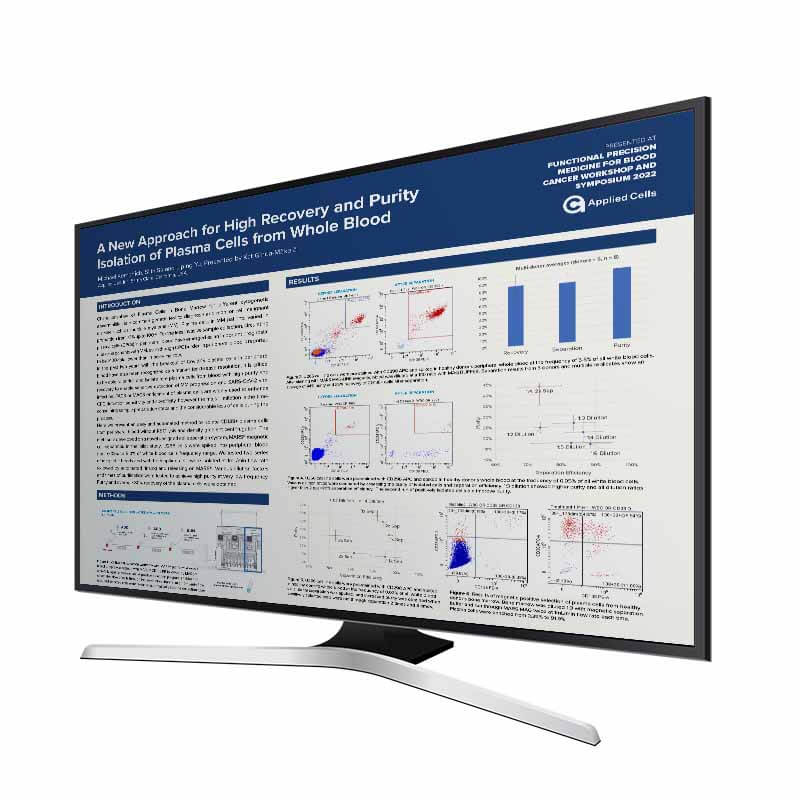
Characterization of Plasma Cells in Bone Marrow for different cytogenetic abnormalities is a common genetic test to diagnose and monitor cell malignant disease such as multiple myeloma (MM). Plasma cells in MM patients varied in proportion from <1% up to 100%. For the less invasive sample collection, circulating plasma cells (CPCs) in peripheral blood have emerged as an important prognostic marker in patients with MM, even though CPC burden in peripheral blood is reported to be >100-fold lower than in bone marrow.
In the past two years with the breakout of Covid-19 plasma cells in peripheral blood have also been recognized as a marker for disease resolution. It is critical to be able to enrich and isolate rare plasma cells from blood with high purity and recovery to enable sensitive detection of MM progression and SARS-CoV-2 viral infection. FACS or MACS enrichment of plasma cells are widely used to enhance CPC detection sensitivity and specificity, however the major limitation is the timeconsuming sample preparation steps and the considerable loss of cells during the process.