GoFast™: accelerating the future of CAR-T cell therapy
Enrich, harvest, and accelerate CAR-T development with a GMP-ready GoFast™ workflow – powered by the MARS® Bar platform to deliver CAR-T cells in under 48 hours.
Ready to GoFast™? Contact us!
GoFast™ – join us in our mission
Our mission is to enable scalable cell therapy manufacturing — with a vision to help reduce manufacturing COGS
below $10,000 with GoFastTM workflow.
Traditional CAR-T manufacturing takes 2–3 weeks, often delaying treatment for critically ill patients. GoFast™ reduces this timeline to under 72 hours, enabling faster delivery of life-saving therapy when every moment counts. Its streamlined, GMP-ready workflow also supports point-of-care manufacturing, paving the way for decentralized, more accessible treatment solutions.
WHY GoFast™ ?
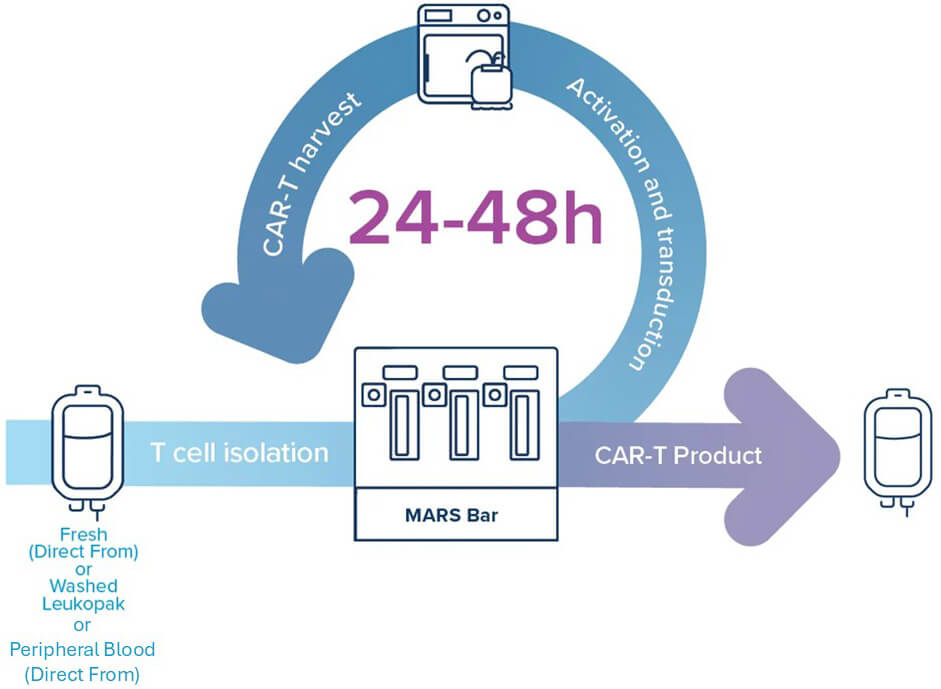
A 3-Step GoFast™ CAR-T Manufacturing
1. T Cell Isolation
- 90% purity, >70% T cell recovery
- Column-free, sterile closed system
2. Activation and Transduction
- High expression in <24 hours
- No extra buffer exchange needed
- Direct from media to transduction
3. CAR-T Harvest
- High yield, high viability
- <1% viral residue, <0.2% B cell contamination
- Formulation-ready or cryopreservation-compatible
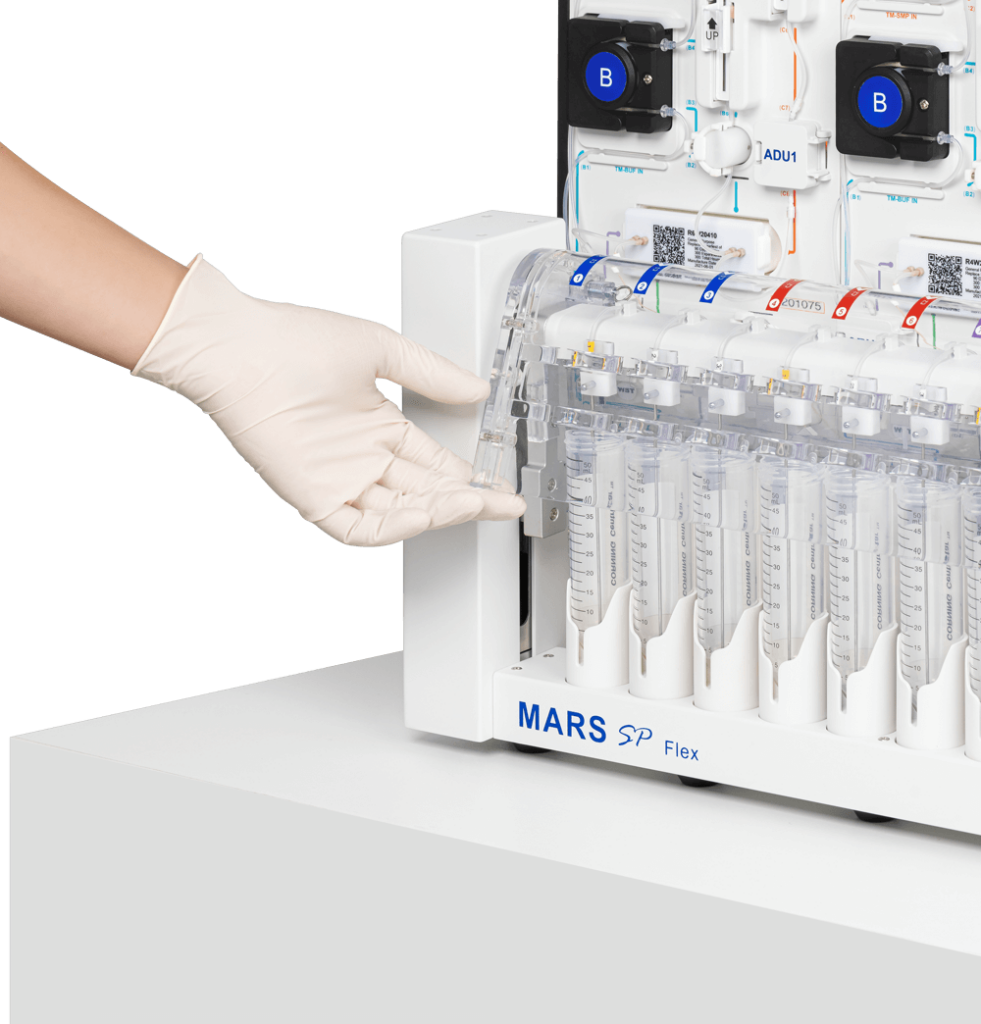

AUTOMATION MEETS INNOVATION
MARS® Bar – one platform for both T cell enrichment and CAR-T cell harvest
- Column-free, closed, sterile fluidics system
- ISO 13485:2016 certified and GMP-ready
- Compatible with fresh or frozen leukopak
- Solution optimized for Applied Cells’ IngenuityTM reagents
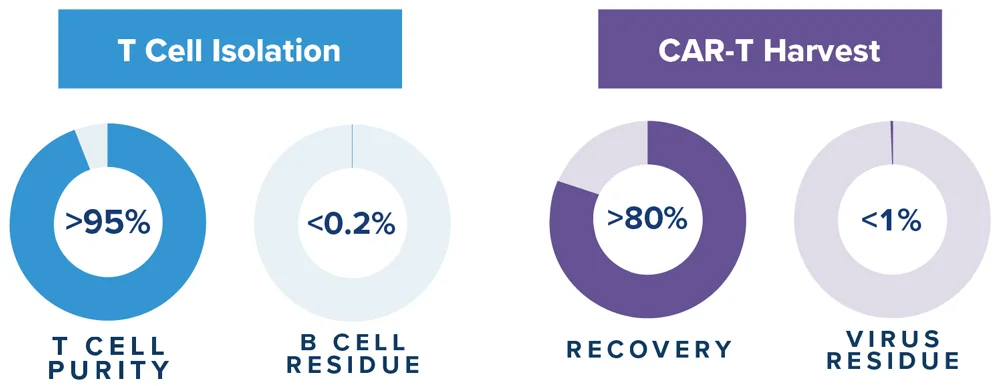
GoFast™ Results
- <72-hour workflow
- >90% purity and >70% T cell recovery
- Low viral / B cell residue
- Lower cost of goods
- High naïve stemness phenotype
- Scalable for point-of-care manufacturing
Discover All Our Products
MARS® SP
Gentle acoustic debris removal for single-step, label-free cell or nuclei enrichment for up to 3 samples with ease and precision.
MARS® Bar
Positive and negative immunomagnetic cell selection with column-free technology for up to 3 samples simultaneously.

Reagents
Broad reagent selection designed and validated for exceptional purity, recovery, and viability using MARS Platform.



