New Year. GoFast™ Year.
GoFast™ – join us in our mission
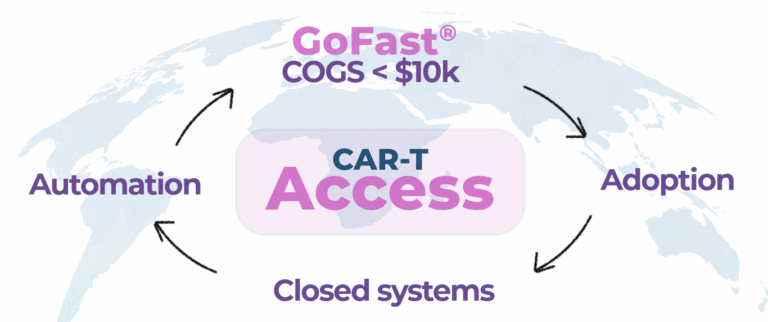
Our mission is to enable scalable cell therapy manufacturing — with a vision to help reduce manufacturing COGS below $10,000 with GoFastTM workflow.
CAR-T cell therapy has demonstrated its clinical value across multiple hematologic malignancies and continues to expand into new indications. The science is no longer the limitation.
The next challenge is access: how quickly, how reliably, and how affordably CAR-T can be manufactured for patients around the world.
The GoFast™ workflow was created to address this challenge directly.
WHY THIS IS THE GoFast YEAR
CAR-T works.
Affordability is the next frontier.
CAR-T is changing its pace. This shift is not just about speed, it is about:- Expanding capacity
- Enabling decentralized production
- Reducing manufacturing cost
- Supporting broader patient access

With manufacturing targets moving toward COGS < $10,000 and a long-term vision below $7,000, this year marks a turning point in how CAR-T can scale. CAR-T works. Now the responsibility is to make it affordable, faster, and more accessible. If affordability, speed, and access are part of your strategy, this may be your GoFast year.
THE GoFast™ PROMISE
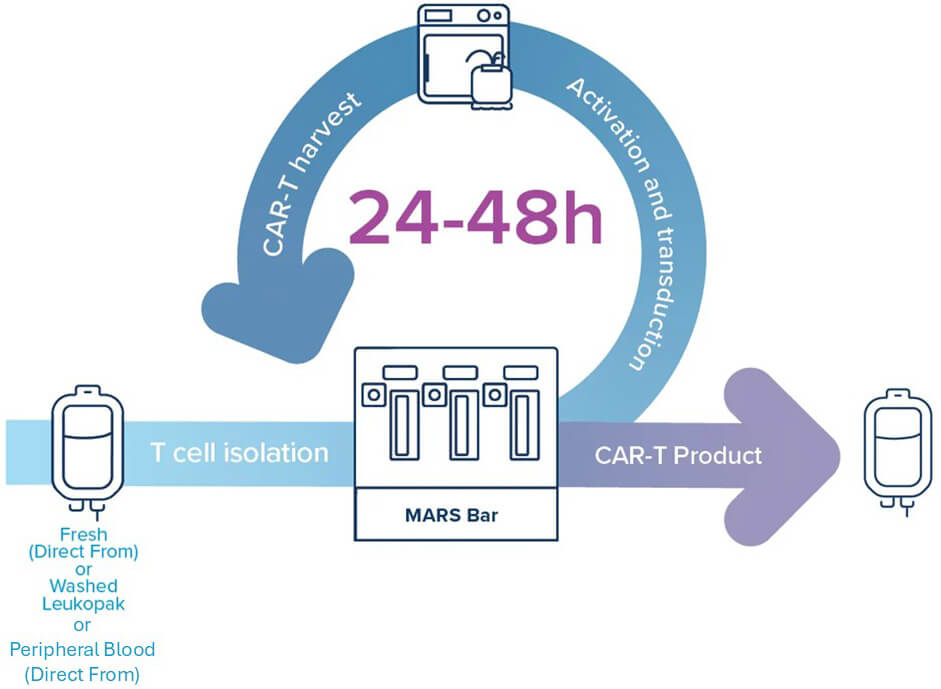
A 3-Step CAR-T Manufacturing
1. T Cell Isolation
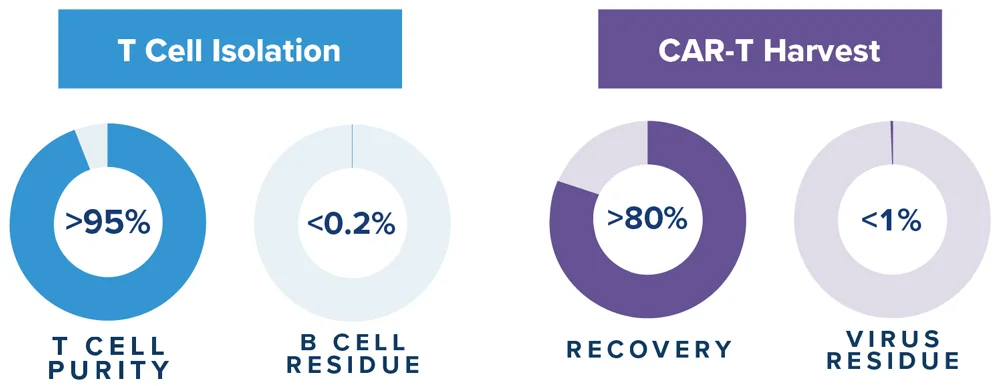
- 90% purity, >70% T cell recovery
- Column-free, sterile closed system
2. Activation and Transduction
- High CAR expression in <24 hours
- No extra buffer exchange needed
- Direct from media to transduction
3. CAR-T Harvest
- High yield, high viability
- <1% viral residue, <0.2% B cell contamination
- Formulation-ready or cryopreservation-compatible

AUTOMATION MEETS INNOVATION
MARS® Bar – one platform for both T cell enrichment and CAR-T cell harvest
The GoFast™ workflow is powered by the MARS® Bar platform:
- Column-free, closed, sterile fluidics system
- ISO 13485:2016 certified and GMP-ready
- Compatible with fresh or frozen leukopak
- Solution optimized for Applied Cells’ IngenuityTM reagents
- Enables both T cell enrichment and CAR-T harvest on one platform

GoFast™ performance
- <72-hour workflow
- >90% purity and >70% T cell recovery
- Low viral / B cell residue
- Lower cost of goods
- High naïve stemness phenotype
- Scalable for point-of-care manufacturing
Discover All Our Products
MARS® SP
MARS® Bar




