T cell isolation from leukopak
Accelerating cell therapy manufacturing with fast, direct processing
Simplified, high-yield T cell isolation process directly from leukopak
In CAR-T and other adoptive cell therapies, manufacturing success is often associated with efficient T-cell isolation. Leukopaks are enriched leukocyte products collected with leukapheresis process and they provide a concentrated source of starting material. However, the T-cell isolation process from leukopaks frequently requires multiple washing steps, which can lead to time consumption, complex workflows, and the risk of cell loss.
The MARS® Bar platform with Ingenuity™ 50 nm CD4 and CD8 magnetic beads enables rapid, high-purity T-cell isolation directly from leukopak, minimizing handling and preserving cell health.
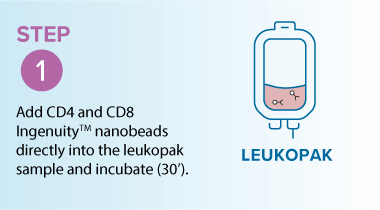
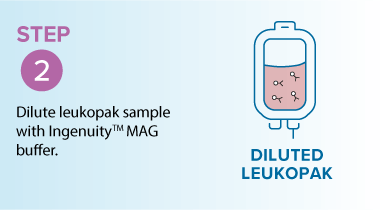

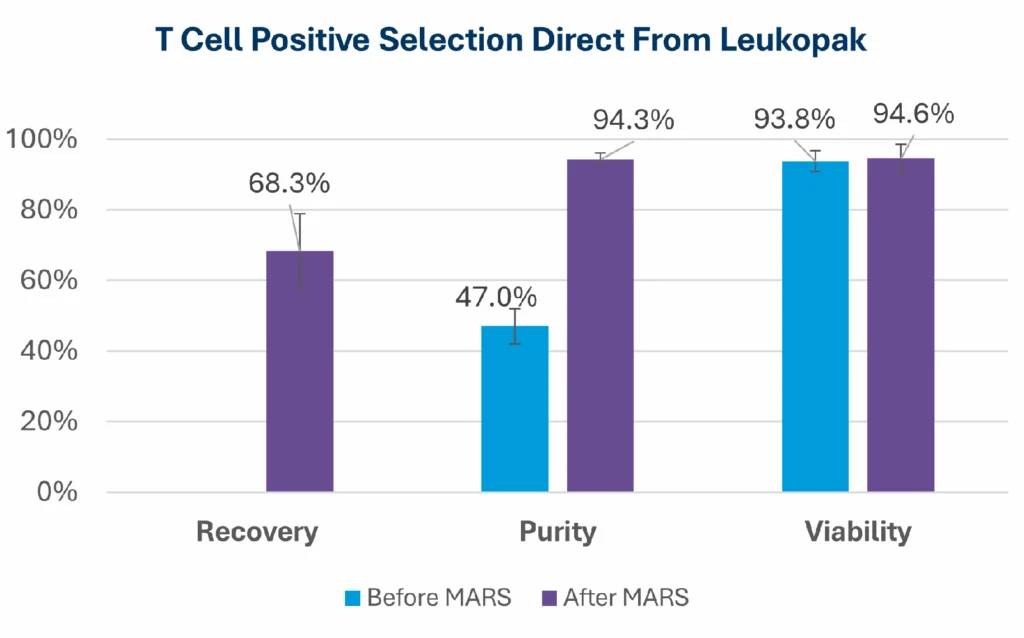
In the example below, starting from a leukopak, the MARS® Bar workflow was used to isolate T-cells 47.0% to 94.3% purity, while maintaining high viability (~94.6%) and delivering high recovery (68.3%).
Advantages of MARS® Bar direct from leukopak T-cell isolation are:
Cost-effective — no centrifugation steps; fast separation time
Efficient — high purity , yield and cell viability
Closed fluidics — GMP compliance
By integrating MARS Bar T-cell isolation directly from leukopak protocol into CAR-T manufacturing workflows, it is possible to increase manufacturing throughput, improve consistency, and reduce costs of the therapies – making them more accessible to patients worldwide.


AUTOMATION MEETS INNOVATION
MARS® Bar – one platform for both T cell enrichment and CAR-T cell harvest
- Column-free, closed, sterile fluidics system
- ISO 13485:2016 certified and GMP-ready
- Compatible with fresh or frozen leukopak
- Solution optimized for Applied Cells’ IngenuityTM reagents