T cell isolation from washed leukopak
Reliable T cell isolation for consistent therapy manufacturing
Simplified, high-yield T cell isolation from washed leukopak
In the rapidly evolving field of adoptive cell therapies, such as CAR-T, the quality of the starting material is critical to the success of downstream manufacturing processes. Leukopaks, which are enriched collections of leukocytes, serve as a valuable source of T cells. However, they often contain contaminants like platelets, plasma proteins, and cellular debris that can interfere with bead-based labeling and reduce T cell recovery. By incorporating a washing step prior to T cell enrichment, these impurities are effectively removed, resulting in a cleaner input material that enhances the efficiency, consistency, and yield of T cell isolation.The MARS® Bar platform, together with Ingenuity™ CD4/CD8 nanobeads, offers a state-of-the-art solution for rapid, high-purity T cell isolation from washed leukopaks. This closed, automated system is ISO 13485:2016 certified, ensuring compliance with regulatory standards while delivering reproducible results for clinical applications.
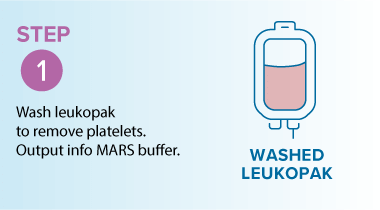
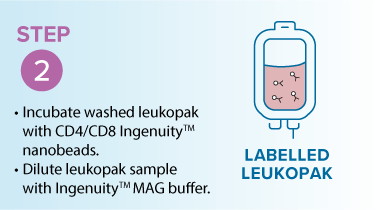
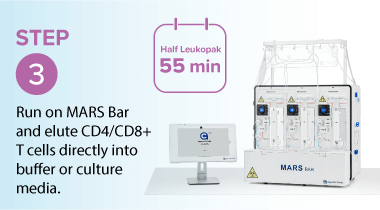
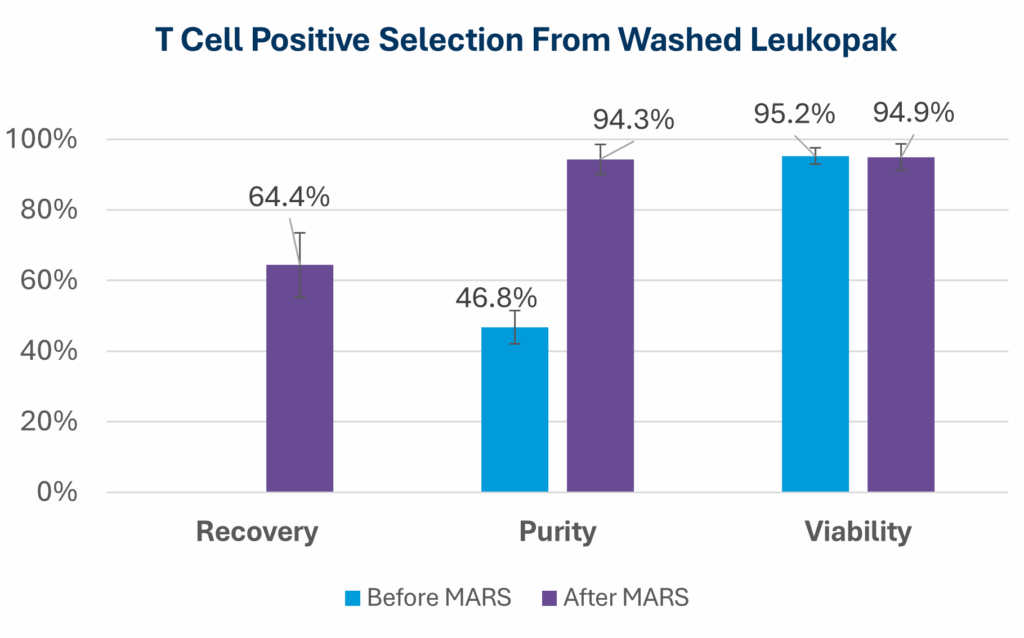
Washing leukopaks prior to T cell selection provides benefits that streamline manufacturing and improve outcomes. By removing contaminants that can non-specifically bind to magnetic beads, washing ensures a cleaner input material for downstream processing. This leads to improved purity and yield, with pre-washed leukopaks enabling higher T cell recovery and purity—for example, studies using the MARS® Bar platform have achieved over 94% CD3⁺ purity across donor samples (see below)1. In addition, gentle automated washing systems like the LOVO cell processing system minimize cell stress and preserve viability, which is critical for robust T cell expansion and functionality in therapies. Finally, washing helps reduce donor-to-donor variability by standardizing the starting material, resulting in more consistent and predictable outcomes.
The integration of a leukopak washing step with the MARS® Bar platform offers significant advantages for CAR-T and other adoptive cell therapies:
- Cost-effectiveness – by reducing non-specific binding, washing decreases the amount of nanobeads required, lowering overall costs
- Efficiency – MARS® Bar platform delivers high T cell recovery, purity, and high cell viability, optimizing the yield of functional T cells.
- Consistency – the combination of washing and automated T cell isolation minimizes variability, ensuring reproducible results across diverse leukopak samples.
By integrating a leukopak washing step with the MARS® Bar platform and Ingenuity™ CD4/CD8 nanobeads, manufacturers can achieve very high T cell isolation performance. This approach delivers high-purity, viable T cells with reduced variability and cost, providing more efficient and scalable production of CAR-T and other adoptive cell therapies.
1. Langa P, Sharma K, Sellers DL, Placencia V, Smith EA, Fick D, et al. Enrichment of CD4+ and CD8+ T-Lymphocytes with a Column-Free Flow Based Device for Clinical Cell Manufacturing. Cytotherapy. 2025. Available from: https://doi.org/10.1016/j.jcyt.2024.12.009


AUTOMATION MEETS INNOVATION
MARS® Bar – one platform for both T cell enrichment and CAR-T cell harvest
- Column-free, closed, sterile fluidics system
- ISO 13485:2016 certified and GMP-ready
- Compatible with fresh or frozen leukopak
- Solution optimized for Applied Cells’ IngenuityTM reagents