T cell Isolation from Whole Blood
A Faster, Simpler Path to CAR-T Manufacturing
Simplified, time-saving T cell isolation process directly from Whole Blood
Chimeric antigen receptor (CAR) T-cell therapy has changed the treatment landscape for hematologic malignancies, offering life-saving potential for patients. Yet one of the biggest obstacles to making CAR-T therapy more accessible is the first step in the manufacturing process, collecting enough high-quality T cells.
Traditionally, CAR-T manufacturing begins with leukapheresis, a procedure in which a patient’s blood is circulated through an apheresis machine to separate mononuclear cells (MNCs) while the rest of the blood is returned. Leukapheresis requires specialized, costly equipment, a trained medical team to supervise the procedure, and specialized facilities that often mean long-distance travel for patients. Additionally, leukapheresis can cause side effects such as citrate toxicity or vascular injury.
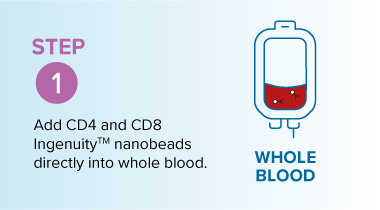
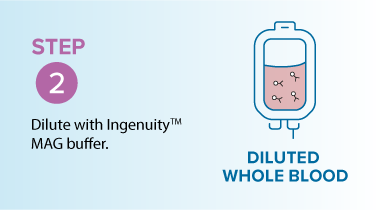

In resource-limited settings where access to equipment, staff, and infrastructure is constrained, direct-from-whole-blood T-cell isolation can offer a faster, more effective solution. The MARS® Bar platform with Ingenuity™ 50 nm superparamagnetic beads enables the direct isolation of T cells from whole blood, with no need for Ficoll separation or lengthy centrifugation steps.
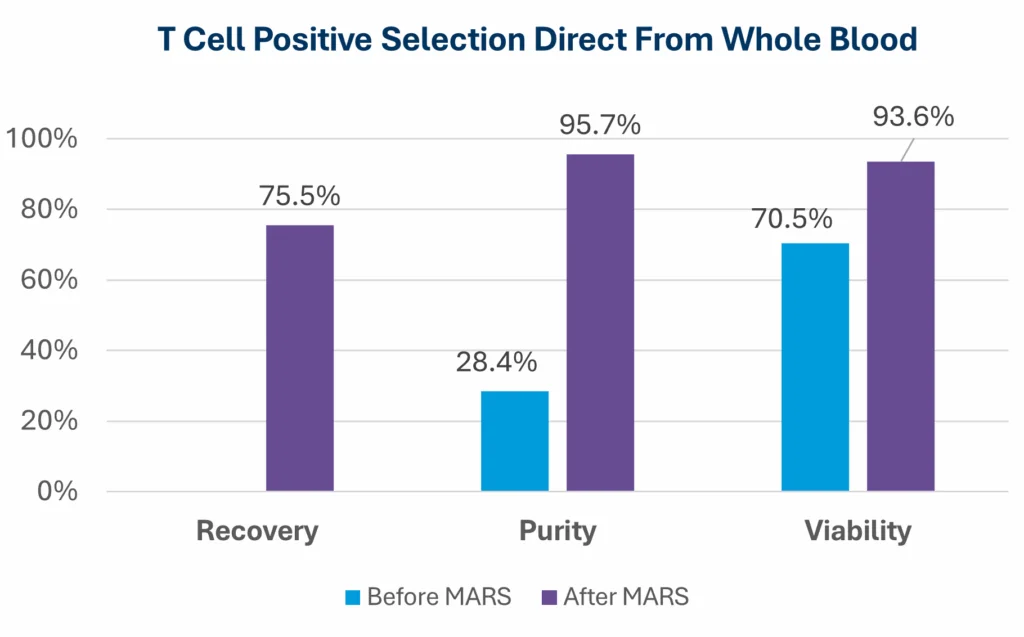
In the example below, using Ingenuity™ CD4 and CD8 nanobeads for positive selection starting with an average T-cell purity of 28% in whole blood, the MARS® Bar platform delivered an average recovery of 75% with a mean final purity of 96%. Using the MARS® Bar platform, viability in the sample improves after isolation.
The MARS® Bar T-cell isolation directly from whole blood process delivers several advantages:
– Speed for fast turnaround, which is critical for patients needing urgent CAR-T treatment.
– Efficiency through reduced number of steps, which lowers the risk of cell loss.
– Cost-effectiveness, by eliminating leukapheresis and multiple purification steps.
T-cell
isolation can be performed in basic healthcare settings, expanding the reach of
advanced cell therapies, making it accessible to more patients.
By integrating high-purity T-cell isolation directly from
whole blood into CAR-T manufacturing workflows, it becomes possible to shorten
vein-to-vein time, reduce costs, and make potentially curative therapies more
accessible to patients worldwide.


AUTOMATION MEETS INNOVATION
MARS® Bar – one platform for both T cell enrichment and CAR-T cell harvest
- Column-free, closed, sterile fluidics system
- ISO 13485:2016 certified and GMP-ready
- Compatible with fresh or frozen leukopak
- Solution optimized for Applied Cells’ IngenuityTM reagents