Exploring the benefits of rapid CAR-T manufacturing for cancer treatment
CAR-T cell therapy has emerged as a revolutionary cancer treatment in recent years, showing promising and relatively long-lasting results. It is especially game-changing for patients with difficult-to-treat and recurring cancers, such as multiple myeloma and certain lymphomas,1,2 achieving complete remission rates of 70-90 percent. 3 The mechanism of, generating CAR-T therapies involves engineering patients’ own T cells to make T cells express “chimeric antigen receptor” (CAR), which can bind to the antigen on cancer cells leading to destruction of cancer cells. CAR-T cells proliferate in patient’s body and are considered to be a “living drug”, which has shown the capability of curing cancer.
Unfortunately, most patients around the globe still won’t get CAR-T at all. These therapies are expensive—half a million dollars in some cases. The manufacturing cost of goods (COG) of autologous CAR-T product is estimated to be $95,780 per dose.6 The conventional manufacturing process for CAR-T therapies is mainly centralized and lengthy, and complex. Thus the vein-to-vein time can range from 3-5 weeks6, which is too much waiting time for patients. To make CAR-T cell therapy fully beneficial to patients, the entire field is working toward lowering the cost and shortening the manufacturing time.
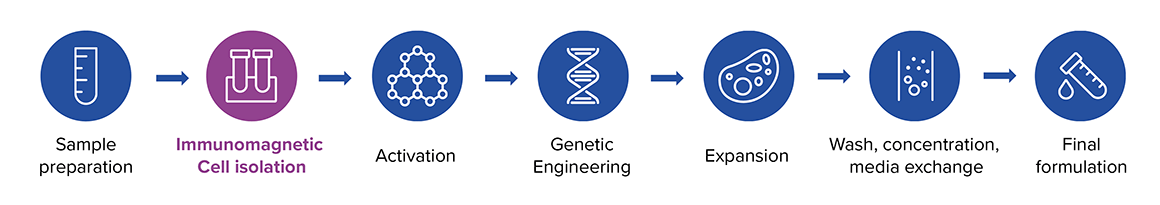
The current manufacturing process involves numerous steps from sample extraction to treatment – including immunomagnetic cell isolation, cell activation, genetic engineering, expansion, washing and concentration, media exchange and further formulation (Figure 1).
This approach demands a substantial number of cells upfront – ideally a leukopak of over 10 billion cells – and the prolonged cell culture to get hundreds of millions CAR-T cells for good dosage. Prolonged ex vivo proliferation can reduce CAR-T cell potency and lifespan, potentially decreasing their therapeutic efficacy and increasing the risk of relapse for specific cancer types.2,3
Taking CAR-T therapy manufacture closer to the patient
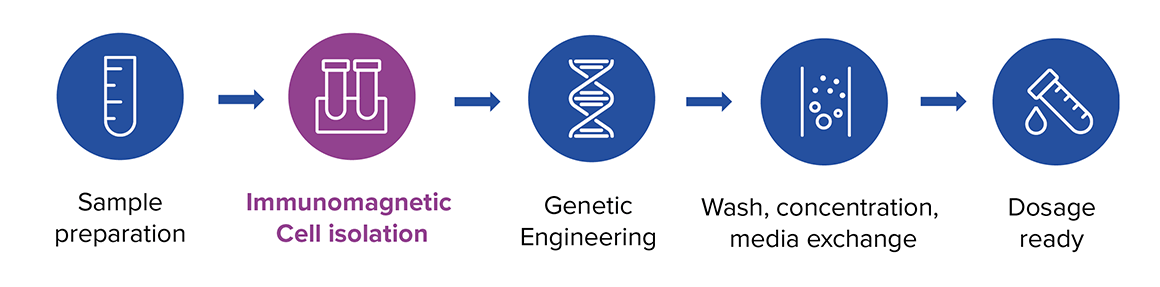
Long lead times and the risk of lower efficacy highlight the need for a novel, decentralized manufacturing process for CAR-T therapies that will improve both the effectiveness and accessibility of these potentially life-changing treatments. A novel rapid CAR-T manufacturing process shows potential to address these hurdles, offering a more efficient and affordable route to small-scale, on-site production.1 This workflow skips the T cell ex vivo expansion stages, streamlining production and reducing the time from leukapheresis to infusion.1
Following a brief transduction period, cells will undergo washing and concentration before being re-infused into the patient, where they proliferate and function (Figure 2). The rapid process of manufacturing CAR-T cells can be as short as 24 hours2,3,4, offering promise to shorten the vein-to-vein time to less than a week when a quick release QC is implemented. Eliminating the culturing steps the rapid process helps reducing the cost and labor.
Improved potency and reduced sample requirements
The new approach reduces T cell exhaustion and results in CAR-T cells that are up to 100 times more potent, while containing only 3 million dosed CAR-T cells.2,4 Additionally, these cells exhibit more stemness, less exhausted phenotype with greater proliferation and killing function compared to conventional CAR-T cells.3 This smaller dose leads to need of fewer cells to start with, as a results leukopak is not the only source for CAR-T manufacturing and peripheral blood being an less invasive and more cost-effective becomes the other source.8
Promising clinical results
In clinical trials, optimized rapid CAR-T therapies have already shown remarkable results, with patients experiencing early, profound remissions, and many achieving long-term remission without needing additional treatments.3 Additionally, CAR-T cell expression can persist for up to six months postadministration, offering more durable effects than traditional CAR-T therapies. 2 This is due, in part, to the maintenance of a ‘memory’ phenotype in the CAR-T cells, which exhibit greater persistence in the body, supporting extended cancer control and improved remission durability.4 On top of this, preclinical and clinical studies demonstrate higher expansion rates both in vitro and in vivo, resulting in a more robust immune response and faster remission. Remarkably, many patients achieve minimal residual disease-negative remission within just 14 days of infusion, underscoring the enhanced efficacy of these therapies.3
Multifaceted advantages for healthcare systems
The list of benefits doesn’t stop there. The rapid manufacturing with smaller doses also leads to lower COGs. With applying innovative solutions to the CAR-T manufacturing COGs per dose can be significantly reduced to below $15,000; less than half the financial expenditure incurred by conventional production methods.5 Localized, rapid CAR-T therapy manufacturing is ideal for hospital settings and by removing logistic steps, could improve responsiveness to patient needs and minimize transit time, which is critical for the efficacy and timely delivery of CAR-T therapies.
An end-to-end solution for decentralized CAR-T production
Recent technological advancements, like closed manufacturing units and automated processes, are crucial to maintaining control and consistency of CAR-T cell therapy manufacture and lowering the cost by reducing labor time. A novel end-to-end platform will be uniquely positioned to complete this new three-step process, enabling streamlined, decentralized rapid CAR-T therapy manufacturing.5 The newly developed MARS® Atlas, is compact, modular platform that covers the entire CAR-T production workflow, from cell isolation, genetic modification to preparation for patient reinfusion – all within 24 to 48 hours. The first module isolates T cells using 50 nm magnetic nanobeads, ensuring precise CD4 and CD8 cell selection. In the second module, transduction occurs within an enclosed culture environment to produce CAR-T cells safely and consistently.
The final module uses either magnetic or acoustic methods to purify CAR-T cells into an intravenous-ready solution or cryopreservation media, eliminating the need for multiple separate devices and reducing costs and space requirements. Efficient manufacturing using this platform enables rapid CAR-T production from peripheral blood or fresh/frozen leukopak, supporting faster, small-scale CAR-T therapy manufacture. This process is being validated with rapid quality control tests for quick product release to enable shortened vein-to-vein time.
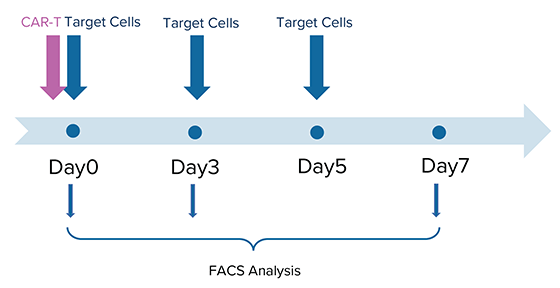
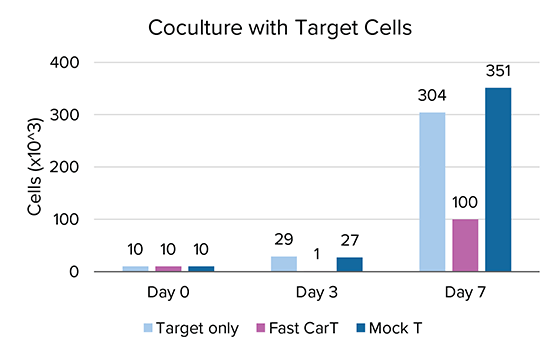
Initial studies have shown that using MARS® Atlas rapid CAR-T manufacturing approach, CAR-T cells demonstrate improved replicative potential, and effectively kill target tumor cells in a seven-day co-culture period (Figure 3).
Paving the way to personalized cancer treatment at the “point-of-care”
Decentralized, small dose rapid CAR-T cell therapy production with integrated, end-to-end closed systems represents a promising new frontier in personalized medicine, addressing a critical need for access to treatment for patients who have not responded to conventional chemotherapies. By bringing CAR-T manufacturing closer to the point of care, healthcare systems can adopt these technologies without large capital investments, making these cell therapies more cost-effective and more accessible. Looking ahead, this trailblazing technology could very well lay the groundwork for future innovations in personalized cell therapies for a broader range of medical conditions.
We are now welcoming initial adopters for this new end-to-end closed manufacturing platform – the MARS Atlas – ahead of the upcoming official product launch. Get in touch with us if you would like to learn more about joining this exciting early adopter program.
References
1. Svoboda J, Landsburg DJ, Nasta SD, et al. Safety and efficacy of armored huCART19-IL18 in patients with relapsed/refractory lymphomas that progressed after anti-CD19 CAR T cells. Journal of Clinical Oncology. 2024;42(16_suppl):7004-7004. doi:10.1200/JCO.2024.42.16_suppl.7004
2. Dickinson MJ, Barba P, Jäger U, et al. A Novel Autologous CAR-T Therapy, YTB323, with Preserved T-cell Stemness Shows Enhanced CAR T-cell Efficacy in Preclinical and Early Clinical Development. Cancer Discov. 2023;13(9):1982-1997. doi:10.1158/2159-8290.CD-22-1276
3. Yang J, He J, Zhang X, et al. Next-day manufacture of a novel anti-CD19 CAR-T therapy for B-cell acute lymphoblastic leukemia: first-in-human clinical study. Blood Cancer J. 2022;12(7):104. doi:10.1038/s41408-022-00694-6
4. Ghassemi S, Durgin JS, Nunez-Cruz S, et al. Rapid manufacturing of non-activated potent CAR T cells. Nat Biomed Eng. 2022;6(2):118-128. doi:10.1038/s41551-021-00842-6
5. Harrison RP, Ruck S, Medcalf N, Rafiq QA. Decentralized manufacturing of cell and gene therapies: Overcoming challenges and identifying opportunities. Cytotherapy. 2017;19(10):1140-1151. doi:10.1016/j.jcyt.2017.07.005
6. Harrison RP, Zylberberg E, Ellison S, Levine BL. Chimeric antigen receptor-T cell therapy manufacturing: modelling the effect of offshore production on aggregate cost of goods. Cytotherapy. 2019;21(2):224-233. doi:10.1016/j.jcyt.2019.01.003.
7. Willyard C. The effort to make a breakthrough cancer therapy cheaper: CAR-T cells could revolutionize the treatment of a wide variety of diseases, if only we can make them cheaper. Biotechnology and Health. 2024 Apr 12. Available at: https://www.technologyreview.com/2024/04/12/1091161/car-t-cancer-therapy-treatment-expense/.
8. Daniel A. et.al., Development of Ingenui-T, a Novel Vein-to-Vein Solution for Rapid Autologous CAR T-Cell Manufacturing Starting From Whole Blood, for the Treatment of Autoimmune Diseases, bioRxiv, January 24, 2024

