The future of Point-of-Care (PoC) manufacturing: bringing cell therapies closer to the patient
Cell therapies, such as CAR-T, have introduced new possibilities in the treatment of selected cancers. But despite their promise and potential, manufacturing timelines and logistical complexity and challenges continue to affect broader access and adoption. Centralized production models may require several weeks between cell collection and infusion, which can cause delays for patients with rapidly progressing or advanced conditions.
Point-of-Care (PoC) manufacturing is an emerging approach that has the potential to address these challenges. By enabling production closer to the patient, supported by evolving technologies and regulatory frameworks, PoC manufacturing may help streamline access to personalized therapies.
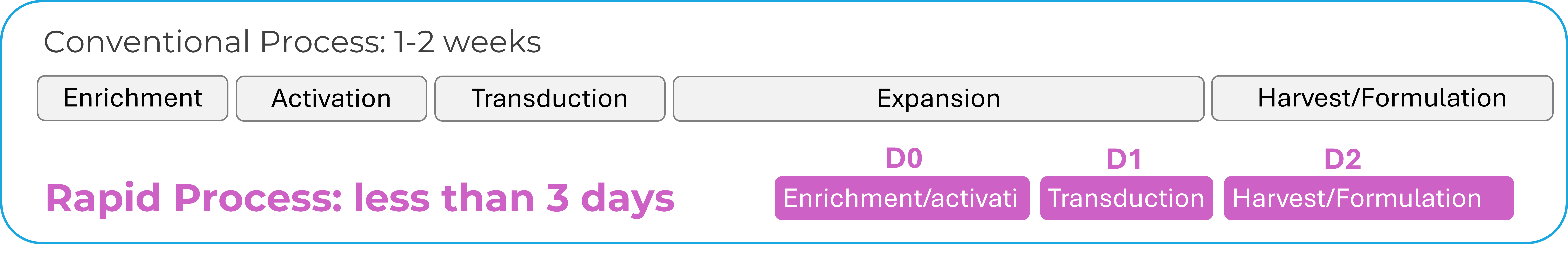
Figure 1: Reducing the vein-to-vein time (i.e. time between leukapheresis and infusion) – can significantly improve patient outcomes.
Benefits of manufacturing closer to patients
Shorter treatment timelines
Reducing the vein-to-vein time (i.e. time between leukapheresis and infusion) – can significantly improve patient outcomes. Traditional centralized manufacturing often requires complex logistics: coordinating cell transport across many locations, managing cold chain logistics, and conducting external quality control (QC). Decentralized manufacturing models, through reducing opportunities for delay or error and improving access, can simplify this process, especially in resource-limited settings 2,3.
Personalization and flexibility
Autologous therapies are inherently variable, because they depend on individual patient’s immune health and treatment history. Point-of-care systems allow clinical teams to adapt processes in real time, improving the probability of success even when working with challenging, variable starting materials2.
Cost-reduction
Decentralized manufacturing can lower costs associated with cell therapy production by reducing reliance on complex logistics, cryopreservation, and large centralized facilities. Automated, closed-system devices used in point-of-care settings further decrease labor and infrastructure expenses, potentially making therapies more affordable.
Improved cell quality by shortening manufacturing time
Studies suggest, that the accelerated cell manufacturing brings benefits such as producing more potent CAR-T cells, reducing production costs and shortening vein-to-vein time.3
In a recent Phase I trial, CAR-T products were manufactured in just three days and administered within five days after apheresis. Despite the short turnaround and reduced cell dose, patients who had previously failed CAR-T therapy showed a 52% response rate1.
Emerging technologies enabling PoC manufacturing
Automation is one of the key enablers of PoC manufacturing. Closed, modular platforms —such as, for example, the MARS® Atlas system — are designed to support GMP-compliant workflows in a compact, hospital-friendly footprint. These systems integrate multiple manufacturing steps: from cell selection, transduction, to expansion and harvest, in a single, walk-away workflow and allow standardized performance across sites and reduce human errors. MARS® Atlas additionally supports the accelerated manufacturing timelines, including the potential for production of one CAR-T cell product within 72 hours from cell collection.
Evolving regulatory frameworks
Regulatory agencies are also beginning to evolve in response the to decentralized models. In the European Union, hospital exemptions provide a path for in-hospital production of advanced therapies under specific conditions. In the United States, the FDA has signaled openness to decentralized production—provided that product quality and comparability of products from different sites are maintained.2
Summary
Point-of-care manufacturing will take time to implement and is expected to complement centralized facilities. However, bringing production closer to patients could open the door to faster, more accessible advanced therapies.
For PoC to be adopted, the regulatory guidance for decentralized sites will need to be harmonized, and new automated, GMP-compliant platforms, such as MARS® Atlas, will need to be adopted. The accelerated manufacturing could reduce the manufacturing cost to further prove the value of PoC.
At Applied Cells, we believe that through collaboration, PoC manufacturing has a real chance to revolutionize how, where, and when patients receive life-saving therapies. Contact us to discuss this topic further.
References
- Svoboda, J. et al. Enhanced CAR T-cell therapy for lymphoma after previous failure. N Engl J Med 2025; 392:1824-35. doi:10.1056/NEJMoa2408771.
- Mackall, C Making cell therapy accessible: challenges and opportunities. Nat Biotechnol 43, 482–486 (2025). doi:10.1038/s41587-025-02625-9.
- Ayala Ceja, M. et al. CAR-T cell manufacturing: major process parameters and next-generation strategies. J Exp Med. 2024;221(2):e20230903. doi:10.1084/jem.20230903.

