How will affordability shape the future of cell therapy?
As cell therapy continues to evolve from hematologic cancers to solid tumors and autoimmune diseases, one key question emerges: how will affordability shape its global future?
While scientific innovation drives the field forward, the next phase of growth will depend on how effectively manufacturing and delivery models adapt to the economic realities of diverse healthcare systems. From established markets to emerging economies, aligning therapy cost with local affordability will determine whether advanced treatments reach patients worldwide, sustainably and equitably.
CAR-T works, now the goal is to make it affordable, for everyone
The science behind CAR-T is no longer theoretical, it is proven. CAR-T therapies have achieved remarkable clinical results in several hematologic malignancies, with durable remissions reported across approved therapies and hundreds of clinical trials.1,2 The field is now expanding rapidly into solid tumors, like brain, lung cancer, stomach, pancreatic cancers, as well as autoimmune diseases such as lupus and myasthenia gravis.3,4,5 In parallel, a new generation of biomarkers and cell-manufacturing analytics is improving patient selection and response monitoring, helping physicians tailor treatment more effectively.6,7
| Indication Area | Example | Reported Outcomes | Reference |
|---|---|---|---|
| B-cell malignancies | Tisagenlecleucel, Axicabtagene ciloleucel | Remission in 40–50% of relapsed/refractory patients | (1,2) |
| Solid tumors | Glioblastoma, gastric & lung cancers; dual-target and logic-gated CAR-T | Emerging efficacy; optimization of tumor-microenvironment targeting continues, responses in gastric/pancreatic cancers and a first complete response in NSCLC | (3, 11,12) |
| Autoimmune diseases | CAR-T for lupus, myasthenia gravis, multiple sclerosis | High remission rates in small cohorts; improved symptom resolution | (4) |
| Biomarkers & analytics | Predictive markers for response and toxicity | Improved patient selection and monitoring accuracy | (5) |
Table: CAR-T works, now the goal is affordability
With these scientific and clinical milestones, the question facing the field is no longer ‘does it work?’ but rather ‘how do we make it available and affordable for everyone who needs it?’
To move from pioneering to scalable therapy, cost and accessibility will now get closer to the center stage.
Affordability as the next frontier with “CAR-T 2.0”
In the first decade of commercial CAR-T therapy, development and delivery were concentrated primarily in the USA and Europe, supported by mature reimbursement frameworks and centralized manufacturing facilities.
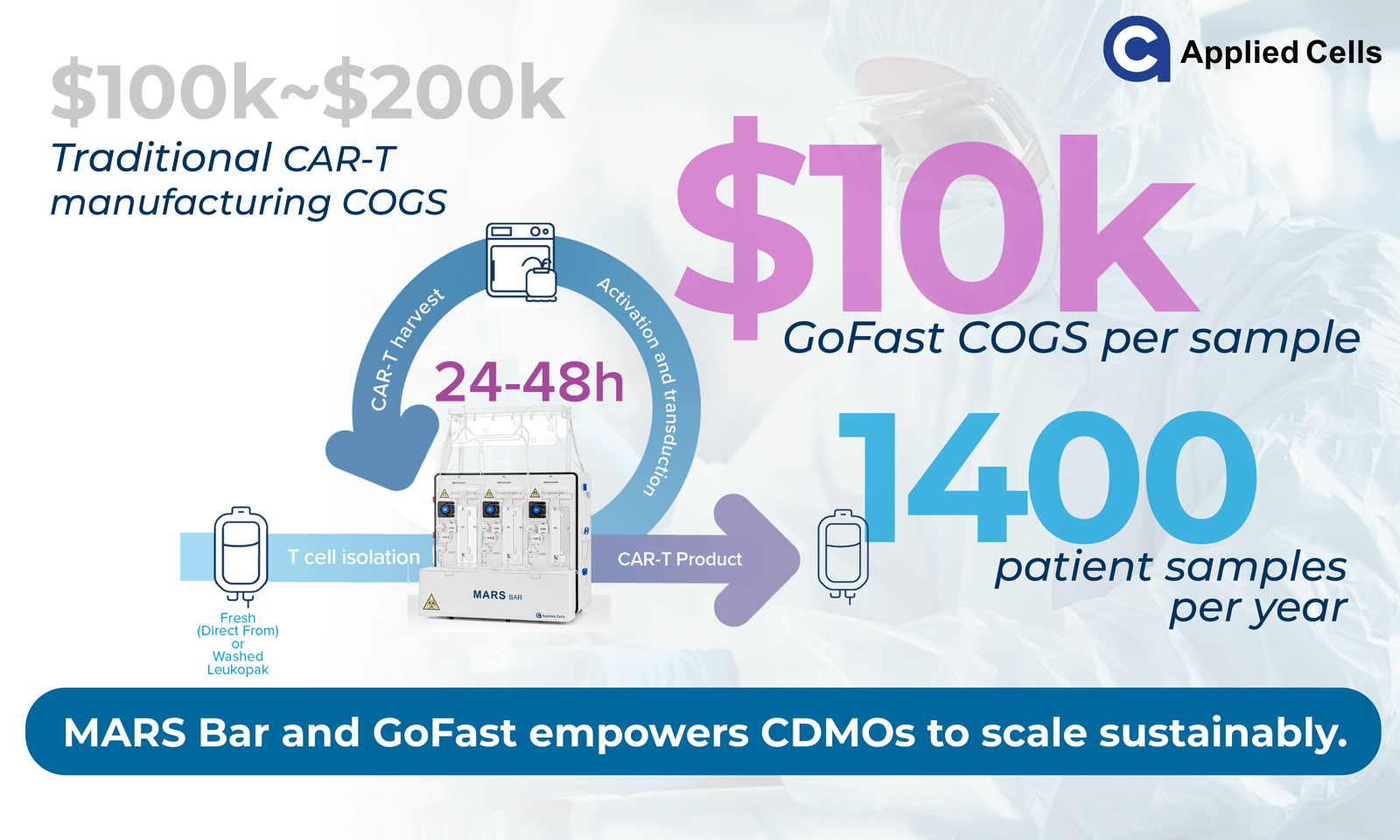
Today, however, opportunity is shifting toward Asia, Latin America, the Middle East, and Africa, where patient demand is stronger than ever but infrastructure and budgets remain constrained. Despite the improvements in process efficiency, manufacturing cost still represents one of the largest barriers to global adoption of CAR-T treatment. Bringing the COGS below the USD $10,000 threshold could make reimbursement and local production feasible across diverse markets.

| United States | China | India |
|---|---|---|
| Approx. 4x ~ 6x the GDP pc | Approx. 30x the GDP pc | Approx. 150x the GDP pc |
Decentralized manufacturing as an enabler
Today, centralized facilities offer scale but carry high infrastructure, labor, and logistics costs. Emerging decentralized manufacturing models, comprising smaller, automated, closed systems located near treatment centers, are helping to reshape this equation.10 They reduce shipping, cryopreservation, and turnaround time, while maintaining process control and quality. Applied Cells’ MARS® Bar platform, when used with the GoFast™ workflow, was developed to support these goals:- Compact footprint: operation within ~10 m² of lab space.
- High throughput: up to five samples per day (targeting ≈1,200 annually, based on internal estimates).
- Minimal infrastructure: MARS® Bar + CO₂ incubator can complete the workflow.
- Reduced COGS: targeting below USD 10 000 per patient sample.
Operational simplicity and quality
To be sustainable, decentralization must also maintain consistency and compliance. Closed, automated systems like MARS® Bar manufactured under ISO13485 limit manual steps, reduce training burden, and standardize outcomes. GoFast™ workflow contains 3 simple steps:- T-cell selection with MARS® Bar
- CAR transduction and incubation
- Harvest with MARS® Bar
From innovation to implementation: a systematic approach
The affordability challenge is not solved by one innovation alone. It will require alignment of technology, process, and business model. Applied Cells contributes to this progress by providing tools and methods that empower developers and manufacturers to achieve order-of-magnitude COGS reductions.
By combining automation, scalability, and cost efficiency, platforms like MARS® Bar with GoFast™ allow therapy developers, CDMOs, and hospitals to implement high-throughput manufacturing without large capital expansion.
The outcome is not only operational efficiency, but a pathway toward accessible, regionally sustainable CAR-T production.
Conclusion
CAR-T has proven that it works, both clinically and scientifically. The next challenge is ensuring that it works economically for every region and patient population. Affordability will define how far and how fast cell therapy can expand globally.
By reducing COGS and simplifying manufacturing, new generation platforms such as MARS® Bar and GoFast™ provide the enabling technology for this transition towards CAR-T 2.0, empowering innovators, hospitals, and CDMOs to bring life-changing therapies within reach of more patients worldwide.
As the science matures, access becomes the new frontier, and affordability, its driving force.
References
- Schuster SJ et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N Engl J Med. 2019;380(1):45–56. https://doi.org/10.1056/NEJMoa1804980
- Rafii S et al. Advancing CAR-T-Cell Therapy in Solid Tumors. Cancers. 2025;17(17):2898. https://doi.org/10.3390/cancers17172898
- Rafii S et al. Advancing CAR-T-Cell Therapy in Solid Tumors. Cancers. 2025;17(17):2898. https://doi.org/10.3390/cancers17172898
- Wu D et al. CAR T-Cell Therapy in Autoimmune Diseases: Prospects and Challenges. Front Immunol. 2025; https://doi.org/10.3389/fimmu.2025.1613878
- Levstek L et al. Biomarkers for Prediction of CAR T Therapy Outcomes. Front Immunol. 2024;15:1378944. https://doi.org/10.3389/fimmu.2024.1378944
- World Bank. GDP per Capita (current US$) – United States (2024). https://data.worldbank.org/indicator/NY.GDP.PCAP.CD?locations=US
- World Bank. GDP per Capita (current US$) – China (2024). https://data.worldbank.org/indicator/NY.GDP.PCAP.CD?locations=CN
- World Bank. GDP per Capita (current US$) – India (2024). https://data.worldbank.org/indicator/NY.GDP.PCAP.CD?locations=IN
- Marseille E et al. Thresholds for the Cost-Effectiveness of Interventions: Alternative Approaches. Bull World Health Organ. 2015;93(2):118–124. https://doi.org/10.2471/BLT.14.138206
- Shah M et al. Promises and challenges of a decentralized CAR T-cell manufacturing model. Front Transplantation. 2023;3:1238535. https://doi.org/10.3389/frtra.2023.1238535
- Qi C, Zhang P, Liu C, et al. A phase I trial of KD-496, a CLDN18.2/NKG2D dual-targeting CAR-T, in patients with gastrointestinal cancers. Ann Oncol. 2025;36(S2):S618. https://www.annalsofoncology.org/article/S0923-7534(25)02434-2/fulltext
- A2 Biotherapeutics Presents Initial Safety and Efficacy Data from Ongoing Phase 1/2 EVEREST-2 Study, Including First Report of a CompleteResponse to CAR T-Cell Therapy in a Patient with NSCLC Newsroom release, November 7, 2025. https://www.a2bio.com/a2-biotherapeutics-presents-initial-safety-and-efficacy-data-from-ongoing-phase-1-2-everest-2-study-including-first-report-of-a-complete-response-to-car-t-cell-therapy-in-a-patient-with-nsclc/
Disclaimer:
This article is provided for informational and educational purposes only. It is intended to share general insights into global trends in cell therapy development, affordability, and manufacturing innovation. Nothing contained herein should be interpreted as medical advice, clinical guidance, investment recommendation, or regulatory direction.
All figures, ratios, and cost estimates — including GDP per capita comparisons, affordability metrics, and cost-of-goods (COGS) thresholds — are approximate and intended for illustrative discussion only. These values may be based on publicly available economic data, rounded calculations, or generalized industry benchmarks, and should not be interpreted as definitive economic analyses or pricing commitments.
Product specifications, performance data, and cost targets related to the MARS® Bar and GoFast™ platforms reflect internal evaluations, prototype configurations, or vendor-reported design goals. Actual results may vary depending on implementation, scale, and local conditions. Applied Cells makes no representation or warranty, express or implied, regarding the completeness, accuracy, or currency of such information.
References to external studies or clinical outcomes (including CAR-T therapies and disease indications) are drawn from independent peer-reviewed publications and are not generated using Applied Cells products unless explicitly stated. MARS® Bar and GoFast™ platforms are for research use only and are not cleared, approved, or authorized by any regulatory agency for clinical use.
Applied Cells, Inc. reserves the right to modify product specifications, performance claims, or documentation at any time without notice. The content is provided “as is,” without warranties of any kind, and Applied Cells assumes no liability for the use, interpretation, or reliance upon the information presented.

