MARS Atlas®
An advanced end-to-end solution for rapid CAR-T cell therapy manufacturing, designed to empower investigators and drug developers with automated production capabilities in a closed system.
Designed to transform the traditional CAR-T manufacturing paradigm by eliminating the need for long cell expansion, reducing costs, and accelerating the delivery of potent, patient-specific therapies.MARS® Atlas – join early access program
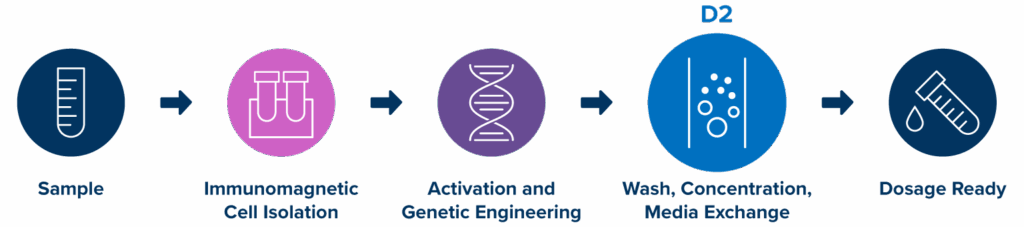
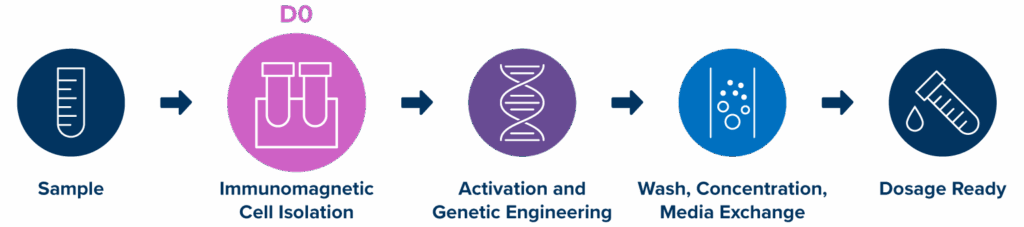
End-to-end workflow
With the three-step workflow and compatibility with peripheral blood, leukopaks, and PBMCs, MARS Atlas represents the future of an accessible, efficient, and flexible CAR-T therapy manufacturing platform.
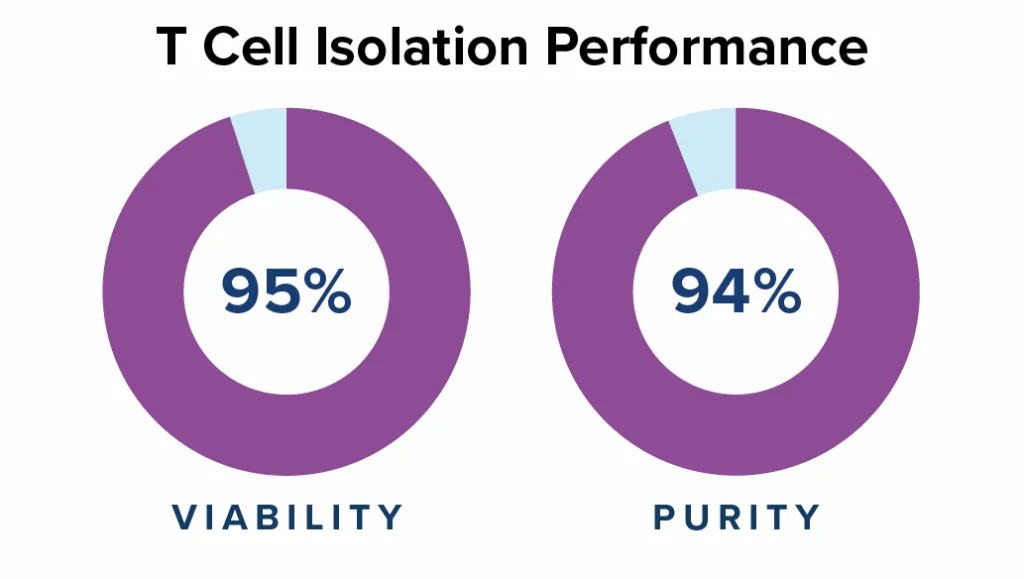
No expansion, high cell quality
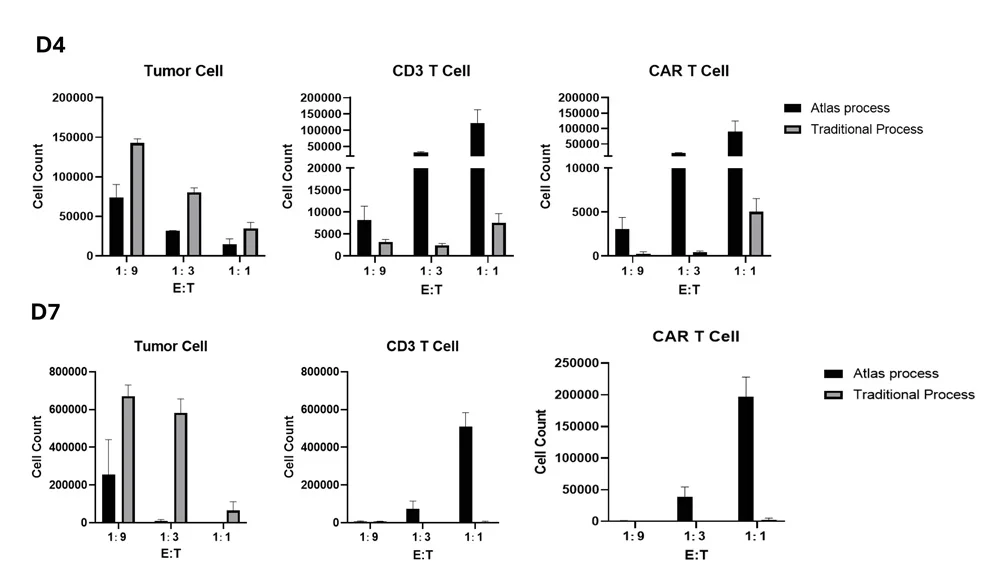
Streamlined manufacturing process bypassing traditional T cell activation and expansion stages reduces T cell exhaustion and preserves cell stemness. The resulting CAR T cells more potent than conventional CAR-T treatments at four weeks post-infusion.
Rapid and simple workflow
A three-step process, Isolation → Transduction → Purification, produces CAR-T cells within 24 to 48 hours. The rapid approach aims to minimize vein-to-vein time, especially important for critically ill patients and point-of-care settings.
Cost-efficient and scalable
Designed to lower the cost of goods (COGs) through reducing labor, logistical demands, and equipment requirements. The compact, modular system supports small-scale, localized production for enabling widespread adoption and personalized cell therapy access.
Towards the future of accessible CAR-T therapies
The field of CAR-T therapy is evolving rapidly, with innovations making these treatments more accessible, efficient, and cost-effective. MARS Atlas represents a pivotal advancement, streamlining CAR-T manufacturing directly at the point of care and supporting new standards for potency, speed, and scalability. With cutting-edge technologies, the platform addresses key challenges in traditional production, driving progress toward the future where personalized cancer care is within reach for more patients.
Learn about the latest advancements in CAR-T therapy and the future of decentralized manufacturing in our new blog post.Example GoFastTM process
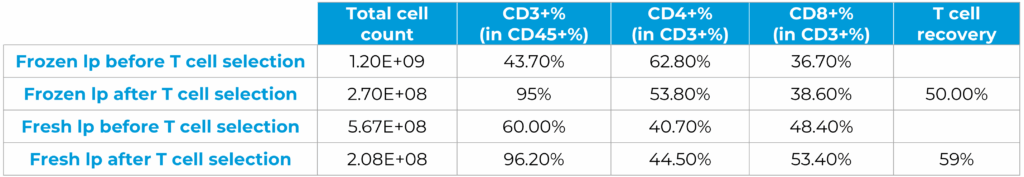

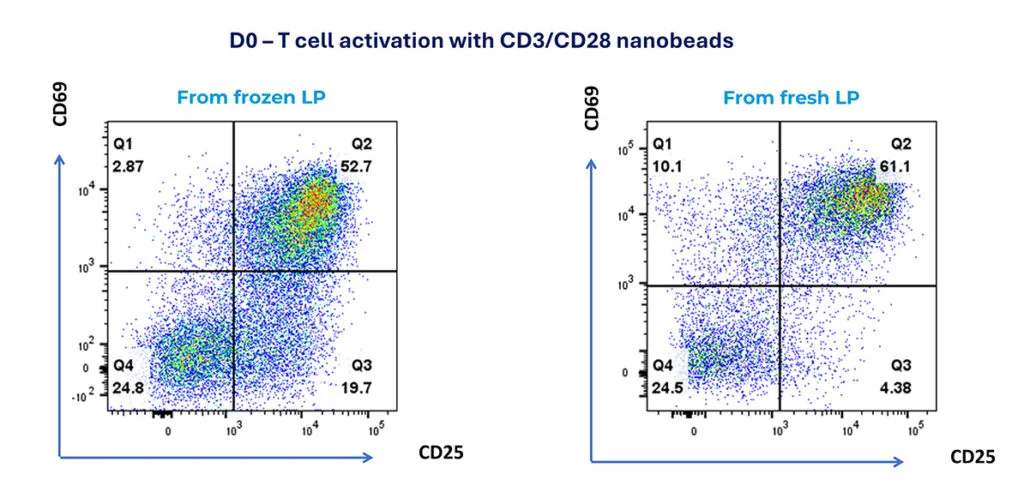
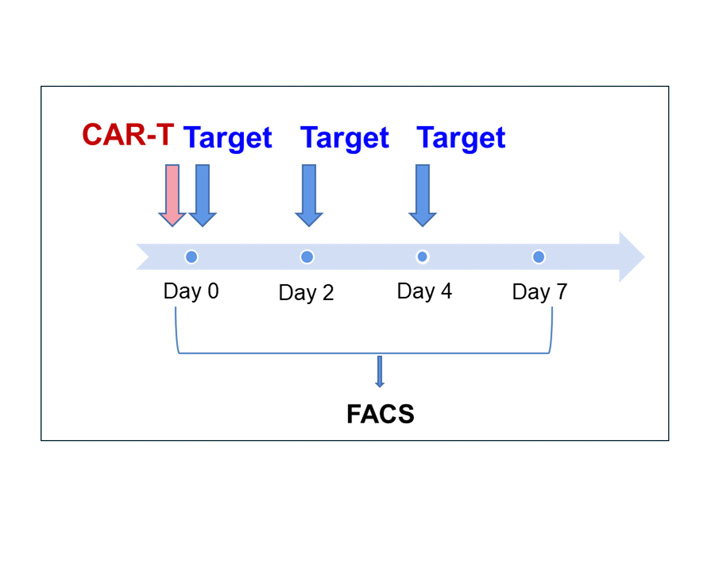
D0 – T cell activation with CD3/CD28 nanobeads
After 24-hour activation, T cell activation was assessed by CD25 and CD69 expression
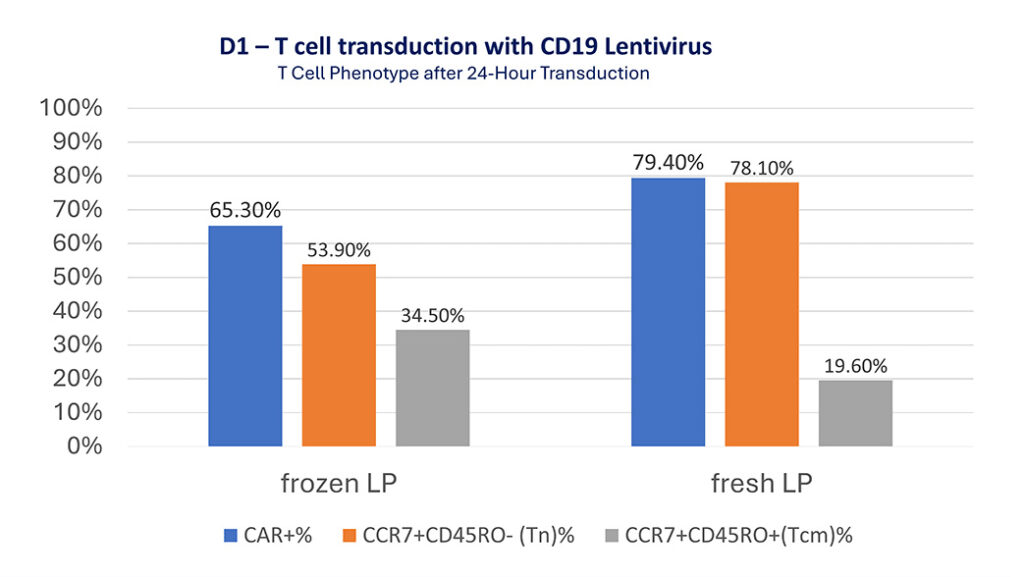
D1 – T cell transduction with CD19 Lentivirus
After 24-hour activation, CD19 lentivirus was added to the flask inside the “transduction module” and incubated for another 24 hours. T cells were analyzed for their phenotypes and CAR+ expression
FAQ
The instrument supports 24 hour process, 48 hour process as well as 72 hour process.
MARS Atlas can process peripheral whole blood, fresh and frozen leukopaks.
The instrument fits both centralized manufacturing and decentralized manufacturing. It can be used in Class C facility, a standard lab in hospitals. This feature makes MARS Atlas an ideal solution for ‘point-of-care’ CAR T manufacturing platform.
Our instrument is the cost-efficient solution, because it enables efficient processing, including fewer steps to get CAR T cells manufactured.
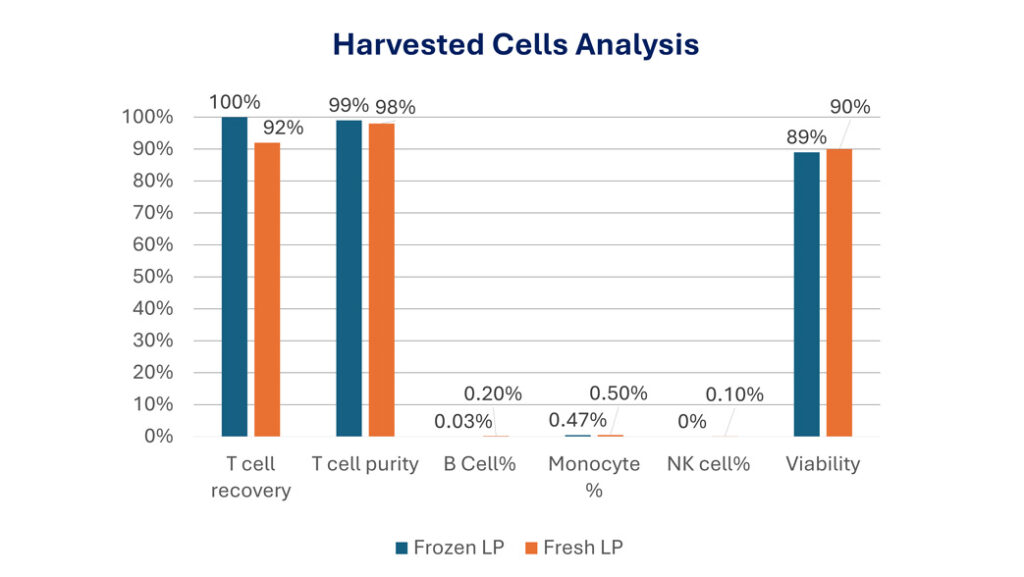
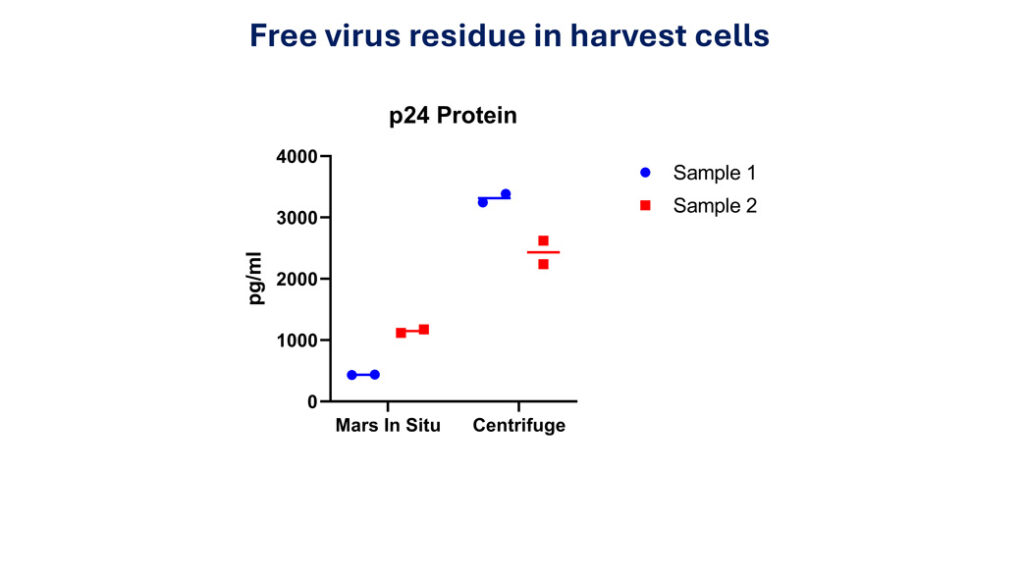
Conventional QC tests take weeks or even months to complete which delays the release of cell product and partially defeats the purpose of developing shortened manufacturing of CAR T cells. We are developing rapid QC test to release CAR T cells, which can be done in two days. On MARS Atlas, CAR-T cells can be harvested in either cryopreservation buffer or storage buffer to allow full characterization of the cells.
Tubing set is sterile certified, reagents are GMP grade. Software is FDA 21CFR11 compliant with audit trail.
Still have questions?
MARS® Atlas – join early access program

Discover All Our Products
MARS® SP
MARS® Bar