Enrichment of multiple myeloma plasma cells for disease monitoring at MGUS and MRD stages

Bone marrow plasma cells characterization is key for diagnosing and monitoring hematologic malignancies, including multiple myeloma (MM). In MM patients, plasma cell numbers can vary, from less than 0.01% to as much as 100% of the total cell population, depending on the stage of the disease. Accurate detection and analysis of plasma cells are especially […]
Enhancing Diagnostic Precision in Multiple Myeloma

Innovative solutions that improve diagnostic precision in multiple myeloma patients have potential to improve patient care and research outcomes. In this study we introduced a streamlined method for isolating CD138+ cells for multiple myeloma (MM) diagnosis and cytogenetic analysis. Using CD138 microbeads and the MARS BAR Flex platform, we achieved high recovery rates, purity, and […]
Efficient CTC enrichment with column-free MARS Bar Platform

Purifying circulating tumor cells (CTCs) is vital for cancer research advancement and new diagnostic and therapeutic strategies. High-purity CTC isolation provides critical insights into tumor biology, metastasis, and treatment response. CTCs are invaluable for personalized medicine development, biomarker discovery, and therapeutic testing. Here, we introduce a rapid and effective CTC isolation method using CD45 depletion […]
Applied Cells releases game-changing reagent kits to empower cell therapy development
We are excited to announce the launch of its MARS® Ingenuity™ Reagent kits for fast and easy cell isolation. This innovative product line features both RUO and GMP-grade reagents, and has been specifically optimized for use on our proprietary MARS® Bar platform. This winning combination offers an all-in-one package for high quality immunomagnetic cell isolation, […]
MARS® Ingenuity™ Line of Reagents brochure

MARS® Ingenuity™ Reagent kits offer comprehensive solutions for the advancement of global cell therapy research, development and manufacturing. Designed with both Research Use Only (RUO) and GMP-grade reagents, these kits provide a fast and straightforward approach to cell isolation, supporting a wide range of therapies including CAR-T, immune cell-based therapies, stem cell-based therapies, and cancer […]
T cell Isolation using CD3/CD28 microbeads
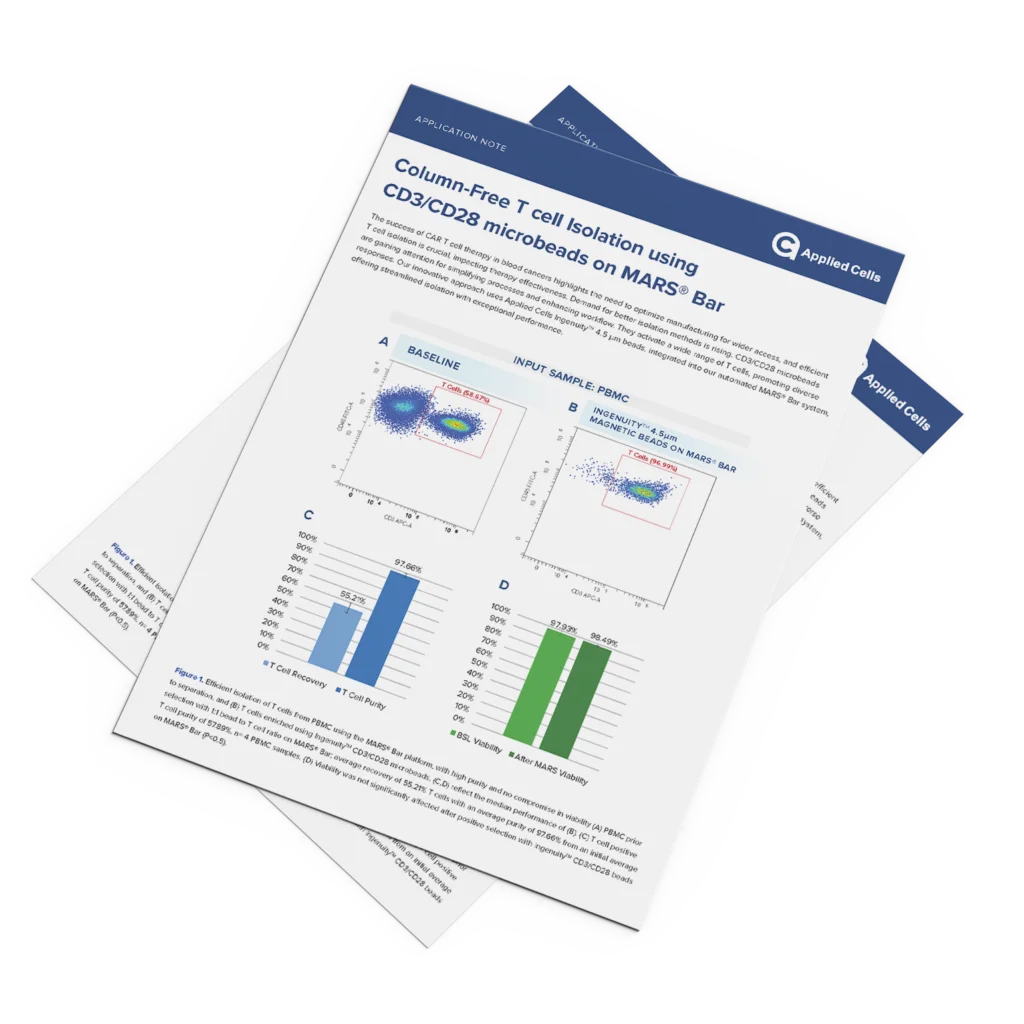
The success of CAR T cell therapy in blood cancers highlights the need to optimize manufacturing for wider access, and efficient T cell isolation is crucial, impacting therapy effectiveness. Demand for better isolation methods is rising. CD3/CD28 microbeads are gaining attention for simplifying processes and enhancing workflow. They activate a wide range of T cells, […]
AACR 2024: A Novel Automated Platform for Rapid Manufacturing of CAR-T Cells
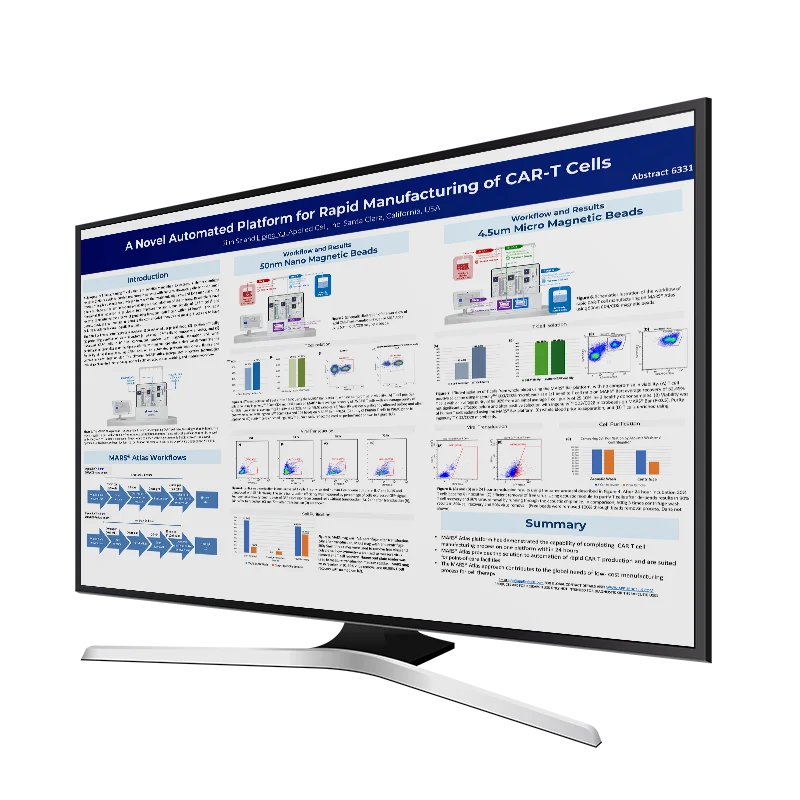
Autologous cell therapy using T cells that are genetically modified to express a chimeric antigen receptor (CAR) has yielded durable responses in patients with cancers. However, globally only a small fraction of eligible patients are privileged to receive the treatment. High cost and long manufacturing time contribute to the key limitations prohibiting a broad adoption […]
Column-Free CD14+ Monocyte Isolation using 50nm Superparamagentic Beads on MARS® Bar

Dendritic cells are professional antigen-presenting cells and they play a key role in the regulation of immune responses. Dendritic cells are widely used as vaccines and drugs for cancer treatment in a large number of trials. Macrophages are essential innate immune cells and when activated, macrophages can mediate the phagocytosis of dangerous cells or materials […]
Column-Free T cell Isolation using 50nm Superparamagentic Beads on MARS® Bar

The therapeutic efficacy of CAR T cells has been firmly established in hematologic malignancies and the key is to develop a low-cost manufacturing process to enable the wide adoption of the therapy. T cell isolation being upstream process for CAR T cell manufacturing has significant impact on the quality of the T cells and the […]
Applied Cells announces first recipients of CellQuest grant at 65th ASH Annual Meeting and Exposition
Applied Cells had the pleasure of attending the 65th American Society of Hematology (ASH) Annual Meeting and Exposition in San Diego, CA, on December 9-12. The team had a wonderful time engaging in insightful discussions on the future of hematology with visitors and taking part in the event’s comprehensive program. This also provided Applied Cells […]

