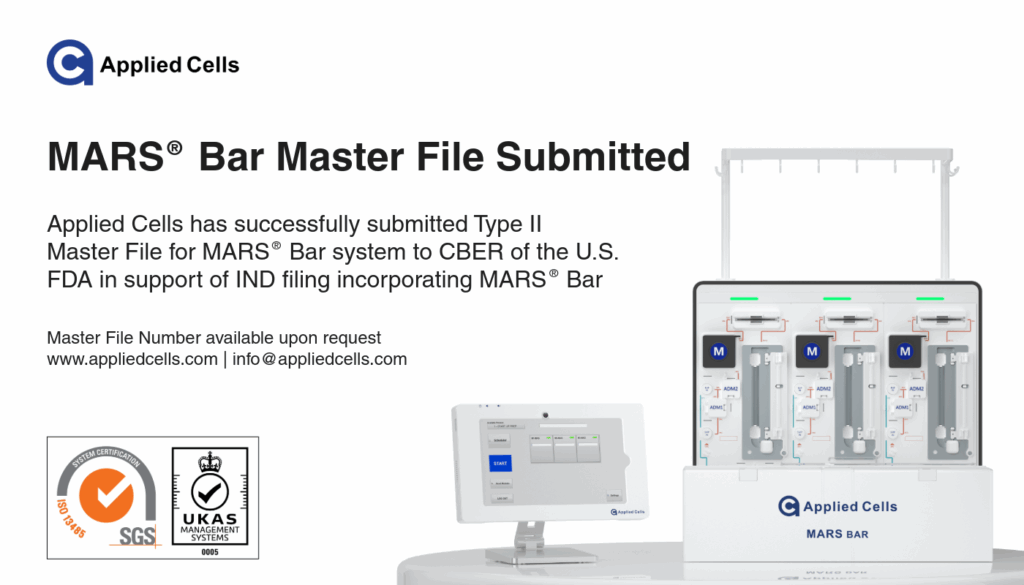
We’re excited to announce another significant milestone — Applied Cells has officially submitted a Type II Master File (MAF) for the MARS® Bar system to the U.S. Food and Drug Administration (FDA), under the Center for Biologics Evaluation and Research (CBER). The Master File Number is available upon request.
This submission reinforces our dedication to advancing cell therapy innovation and supporting our partners’ IND and regulatory pathways worldwide. It’s a key step in demonstrating our ongoing commitment to quality, transparency, and regulatory readiness.
“This Master File submission is a testament to our focus on enabling breakthrough therapies through reliable, regulatory-ready technologies.” — Yuchen Zhou, CEO
This builds on recent achievements including our ISO 13485:2016 certification and the introduction of our Ingenuity™ Line of GMP-grade reagents, which also have Master Files available upon request. We’re proud to be supporting the future of cell therapy with tools that meet the highest standards for manufacturing and compliance, empowering the next generation of life-saving therapies.
Learn more or get in touch with our team at info@appliedcells.com.

