AACR 2024: A Novel Automated Platform for Rapid Manufacturing of CAR-T Cells
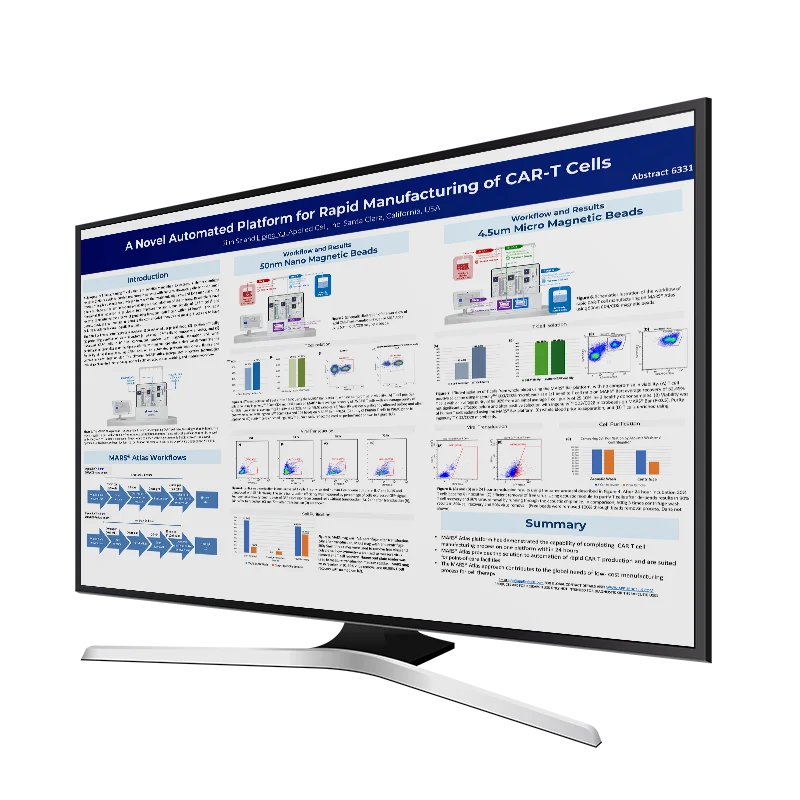
Autologous cell therapy using T cells that are genetically modified to express a chimeric antigen receptor (CAR) has yielded durable responses in patients with cancers. However, globally only a small fraction of eligible patients are privileged to receive the treatment. High cost and long manufacturing time contribute to the key limitations prohibiting a broad adoption […]
ASGCT 2023: Simple magnetic cell separation for both autologous and allogenic cell therapy development
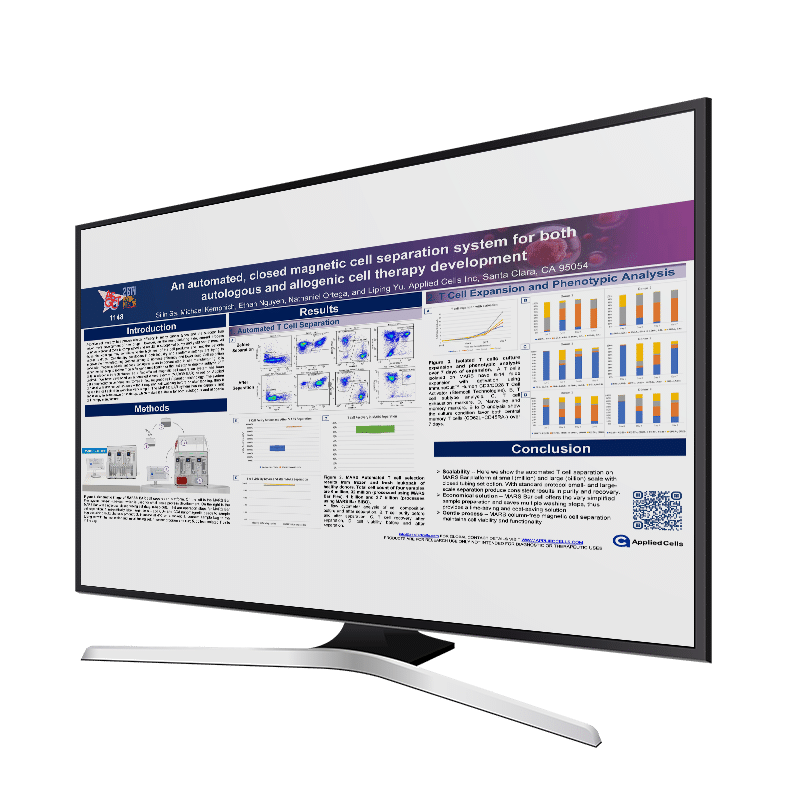
INTRODUCTION Adoptive cell therapy has proven clinical efficacy in many cancer types and the success has driven more development of new drugs. However, on the manufacturing side, current process using conventional tools is complicated and results in suboptimal purity and yield sometimes. All those shortcomings may contribute to the high cost of the cell products […]
Simple magnetic cell separation enabled by an automated, closed system for both autologous and allogenic cell therapy development
INTRODUCTION Adoptive cell therapy has demonstrated effective clinical results in various types of cancer, leading to increased efforts in developing new drugs. However, the current manufacturing process using conventional tools is complex and often yields impure products, contributing to the high cost of cell therapy and limiting patient access. Both industry and academic cell therapy […]
通过无柱免疫细胞磁分选工作流程从 TCR α/ß+中分离TCR γ/δ+

近年来 T 细胞受体 (TCR) 细胞疗法因其为癌症和感染性疾病提供一系列可能性的治疗而获得广泛关注。由于γδ T细胞被视为对癌症免疫治疗颇有潜力的区域细胞,基于γδ T细胞的新型免疫疗法正在被大力开发。尽管TCR疗法在研发、生产及临床应用等领域已经取得重大进展,但 TCR细胞疗法的开发在功效及安全性方面仍然面临很多挑战。 MARS® 平台为优化和扩大 γδ T细胞的研发和生产提供了一种简单而又全新的方法和工具,可以帮助TCR 疗法最大化的发挥临床潜力。
TCR γ/δ+ separation from TCR α/ß+ with MARS® Bar

In recent years T cell receptor (TCR) cell therapy has gained popularity due to a range of possible treatments for cancer and some infectious diseases it could provide. Since γδ T-cells constitute promising effector cell compartments for cancer immunotherapy, novel γδ T-cell-based immunotherapies are being developed. Although advancements in TCR engineering, manufacturing, and clinical applications have led to […]
通过MARS®平台分选 CD3- CD56+ NK 细胞

NK细胞是目前肿瘤免疫治疗领域主要的创新目标。开发和改造基于NK细胞的癌症免疫疗法需要清洁且高效的小体积和大规模的细胞分离方法。创新产品MARS®Bar系统为CD56+/CD3-细胞分离提供了新一代大规模、磁分离技术,可以从单采血、全血或骨髓细胞制品中进行分离。
CD3- CD56+ NK Cell Selection with MARS® Platform

NK cells are currently a major target of innovation in the field of cancer immunotherapy. Efforts aimed at developing and engineering NK cell-based cancer immunotherapy require clean and efficient methods of cell isolation at both small and large scales. The new MARS® Bar system enables a new generation of large scale, magnetic CD56+/CD3- cell isolation […]
Multiphysics innovation for high speed, high recovery, and high viability automated cell separation

INTRODUCTION Tumor biology, immunology, and immune-oncology from multi-omics at the single cell level have witnessed an unprecedented acceleration in recent years, with a major contribution of such speedup from new technologies and products bursting into the market. New technological solutions also contributed deterministically to the promise of cell-based therapy. However, conventional cell separation tools, such […]
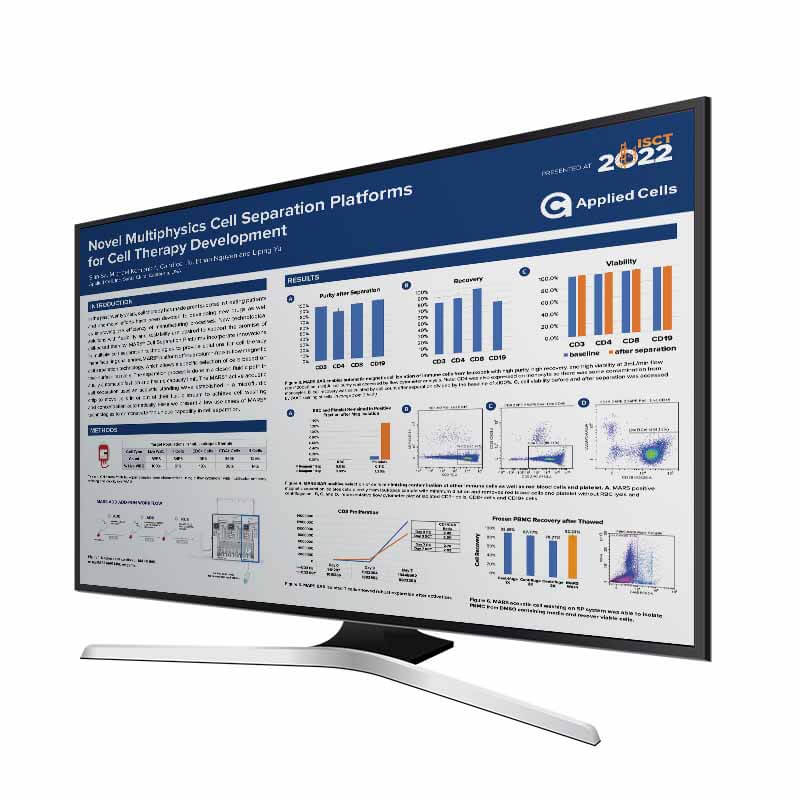
Novel Multiphysics Cell Separation Platforms for Cell Therapy Development

INTRODUCTION In the past twenty years, cell therapy has made great success in treating patients and enormous efforts have been devoted to developing new drugs as well as improving the efficiency of manufacturing processes. New technological solutions with flexibility and scalability are desired to support the promise of cell-based therapy. MARS Cell Separation Platforms incorporate […]
A Novel Platform Providing Automated, Consistent and Gentle Cell Isolation from Tissues for Downstream Applications

INTRODUCTION Tumor-infiltrating lymphocytes (TILs) are lymphocytic cells that have invaded the tumor tissue. Adoptive cell therapy (ACT) of TILs is a strategy to modify the immune system to recognize tumor cells and carry out an anti-tumor effector function. Today, TIL therapies consist of ex vivo expansion of TIL from resected tumor material and adoptive transfer […]

